Impact of obesity and acquisition protocol on 123I-metaiodobenzylguanidine indexes of cardiac sympathetic innervation
Introduction
Patients with heart failure (HF) show augmented activation of the sympathetic nervous system, as reflected by an increase in plasma norepinephrine levels. In addition, neuronal uptake of norepinephrine is impaired in the failing myocardium. Both the enhanced release of norepinephrine and changes in its cardiac neuronal uptake may be responsible for the observed down-regulation of adrenoreceptors in HF (1). Myocardial innervation imaging with 123I-metaiodobenzylguanidine (MIBG) scintigraphy provides a noninvasive tool for the investigation of cardiac sympathetic innervation. Increased norepinephrine turnover and pre-synaptic norepinephrine deficits can be identified by a decreased 123I-MIBG activity quantified as the heart-to-mediastinum (H/M) ratio and an increased 123I-MIBG washout rate from the heart (1,2). Normal cardiac 123I-MIBG distribution includes a relatively low uptake in the inferior wall and extra-cardiac uptake in the liver and lungs may impact image quality and interpretation (3,4). The acquisition of images in prone position might improve the relative tracer uptake in the inferior wall, reducing diaphragmatic attenuation and limiting the liver overlap (5). Also obesity is reported to be associated with sympathetic activation (6-8). Thus, we investigated the relationship between obesity and 123I-MIBG indexes of cardiac sympathetic innervation and if the acquisition protocol influences the results.
Materials and methods
Study population
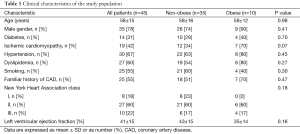
Forty-five patients with HF referred to our Department for cardiac sympathetic imaging were included in the present study. All subjects underwent a complete clinical examination and blood draw for routine biochemical determinations. Demographic data, weight, height, HF medications, New York Heart Association (NYHA) class, tobacco use, hypertension, dyslipidemia, family history of coronary events, presence of diabetes mellitus, presence of co-morbidities, and ischemic versus non-ischemic HF etiology were also collected. The body mass index (BMI) was calculated as weight in kg/height in m2. Obesity was defined as BMI value ≥30 kg/m2 (9). Informed consent, a requirement of the protocol approved by the Institutional Clinical Research Subpanel on Human Studies at our Institute, was obtained in all patients.
123I-MIBG cardiac imaging
All patients underwent 123I-MIBG cardiac imaging according to the recommendations of the Cardiovascular Committee of the European Association of Nuclear Medicine and the European Council of Nuclear Cardiology (10), as previously described in detail (11). An activity of 111 MBq 123I-MIBG was intravenously administered over 1 to 2 min after thyroid blockade by oral administration of 300 mg of potassium perchlorate. Ten-minute planar images of the thorax in standard anterior view (256×256 matrix) were performed 15 minutes (“early” image) and 3 hours and 50 minutes (“late” image) after tracer administration in both supine and prone positions. Imaging was performed using a dual-head camera system (Skylight, Philips) equipped with a low energy, parallel-hole, high-resolution collimator, and the camera peaked at 159 keV with a symmetrical 20% energy window. The H/M ratio was computed from supine and prone images by dividing the mean counts per pixel within the myocardium by the mean counts per pixel within the mediastinum. Using dedicated post-processing software on a dedicated workstation (Philips), the cardiac region of interest (ROI) was polygonal in shape and drawn manually over the myocardium including the LV cavity on the MIBG images. Care was taken to exclude lung and liver from the myocardial ROI. The mediastinal ROI with a square shape was placed on the upper half of the mediastinum and had a size of 7×7 pixels. The location of the mediastinal ROI was determined using as landmarks the lung apex, the upper cardiac border and the medial contours of the lungs.
Image quantification
For both supine- and prone-position acquisition protocols, 123I-MIBG H/M ratios were computed for early and late imaging by dividing the mean counts per pixel within the myocardium by the mean counts per pixel within the mediastinum. The 123I-MIBG washout rate was calculated for both acquisition protocols using the following formula: [(early heart counts per pixel – early mediastinum counts per pixel) – (late heart counts per pixel decay-corrected – late mediastinum counts per pixel decay corrected)]/(early heart counts per pixel – early mediastinum counts per pixel)×100. Using the mean relative uptake, the inferior to anterior uptake ratios were also calculated on early and late images for both acquisition protocols.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and categorical data as percentage. Continuous data were compared by paired or unpaired t test and categorical data by Fisher’s exact test, as appropriate. Multiple linear regression analysis was used to evaluate clinical parameters influencing late H/M ratio. A P value <0.05 (two sided) was considered statistically significant. Statistical analysis was performed with SPSS version 19.0 (SPSS, Inc., Chicago, Illinois, USA).
Results
A total of 45 patients (35 men, age 58±15 years) were included in the study. The clinical characteristics of the patient population are shown in Table 1. The etiology of HF was ischemic in 42% of study population. Twenty-seven patients were in NYHA class II and 10 in NYHA class III. Mean LV ejection fraction was 41%±15% and BMI was 27±4.1 kg/m2. The BMI was <30 kg/m2 in 35 patients and ≥30 kg/m2 in 10 patients. No significant differences in clinical characteristics and in HF medications were detectable between non-obese and obese patients. At multiple linear regression analysis, among clinical variables only BMI (r=−0.03, P<0.05) and LV ejection fraction (r=0.01, P<0.05) were independently related to late H/M ratio.
Full table
123I-MIBG imaging findings
123I-MIBG results of the whole study population are reported in Table 2. As shown, early and late 123I-MIBG H/M ratios, and washout rate were comparable between supine- and prone-position acquisitions. Inferior/anterior uptake ratio in the supine position was significantly lower as compared to prone position on early images (P<0.05). Conversely, inferior/anterior uptake ratio was not different between the two acquisition protocols.

Full table
Impact of obesity on indexes of myocardial innervation
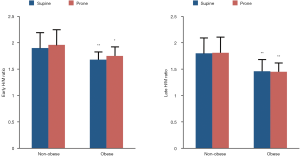
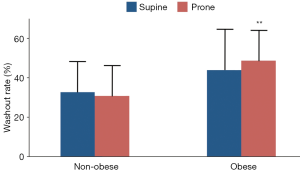
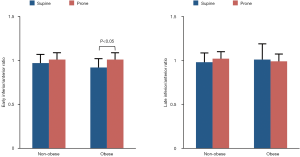
Early and late 123I-MIBG H/M ratios in non-obese and obese patients are depicted in Figure 1. In both subgroups, early and late H/M ratios were comparable between supine and prone positions. Of note, obese patients had lower H/M ratios both in supine (early 1.68±0.13 vs. 1.90±0.32 and late 1.46±0.23 vs. 1.80±0.36, both P<0.01) and prone (early 1.75±0.18 vs. 1.96±0.36, P<0.05 and late 1.45±0.17 vs. 1.81±0.35, P<0.01) positions as compared to non-obese subjects. The washout rate (Figure 2) was higher in obese as compared to non-obese patients in prone position only (48.7±16 vs. 30.8±14, P<0.01). Finally, the early inferior/anterior uptake ratio (Figure 3) in obese patients was lower in supine as compared to prone position (0.92±0.10 vs. 1.01±0.08, P<0.05). To avoid potential gender differences and influence of possible attenuation artifacts, a separate analysis was performed including only men. Obese men had lower late H/M ratio both in supine (1.41±0.17 vs. 1.68±0.30, P<0.01) and prone (1.41±0.13 vs. 1.69±0.24, P<0.005) positions compared to non-obese. The washout rate was higher in obese men compared to non-obese in both supine (48.6±15 vs. 34.4±17, P<0.05) and prone position (52.3±12 vs. 32.8±14, P<0.001).
Discussion
Currently 123I-MIBG is the most widely used tracer for assessing sympathetic nervous function in HF patients and has been also used for risk stratification. Because of the suboptimal 123I-MIBG image quality with single-photon emission computed tomography (SPECT), most of studies have used as a prognostic marker global left ventricular 123I-MIBG uptake H/M ratio calculated on planar supine images. It has been showed that 11C-hydroxyephedrine (HED) positron emission tomography (PET) produces consistently better image quality than MIBG SPECT (4). Furthermore, late MIBG SPECT overestimates the defect area particularly in the inferior and septal regions as compared with 11C-HED PET (4). New multimodality imaging systems, such as SPECT/CT, bring together anatomical and molecular information and provide a means to correct for attenuation effect (12). It has been recently demonstrated that cardiac 123I-MIBG counts can be measured on the tomographic slices within the boundaries of the heart delineated by the CT component even in cases in which tracer uptake is very low (13). If confirmed by further studies cardiac 123I-MIBG SPECT/CT may represent an accurate tool for early diagnosis and monitoring response to treatment in patients with HF.
Several studies have indicated that 123I-MIBG cardiac uptake is reduced in the inferior region also in normal subjects, mainly due to the extra-cardiac uptake by the liver and lungs (3,4,14). Thus, obesity as well as diaphragmatic attenuation, which may occur also for technical reasons, might cause a reduced uptake in the inferior region. Previous studies have demonstrated that regional sympathetic abnormalities assessed by SPECT (15,16) or PET (17) can identify myocardial regions that may be linked to lethal ventricular arrhythmias. The critical issue in the measurement of regional abnormalities on the SPECT is the image quality. Therefore, regional analysis is suitable only if image quality is acceptable. Matsunari et al. (4) reported that the quality of 11C-HED PET images is more suitable for assessment of regional abnormalities than 123I-MIBG SPECT. However, the authors showed a good correlation between late 123I-MIBG H/M ratio and 11C-HED retention. These findings support the use of semi-quantitative H/M ratio data to estimate cardiac sympathetic innervation. Yoshinaga et al. (5) more recently investigated whether prone-position acquisition improves 123I-MIBG image quality in subjects with a BMI <30 kg/m2 by comparing the results to those obtained using supine-position acquisition and high-quality 11C-HED PET/CT. The authors showed that prone 123I-MIBG SPECT significantly reduced heterogeneity of tracer uptake and improved inferior activity in comparison with supine imaging, providing data closer to those obtained with 11C-HED PET. The improvement in inferior uptake may be related to reducing diaphragmatic attenuation and avoiding intense uptake by the liver.
The results of our study, using 123I-MIBG planar scintigraphy, show that the prone-position acquisition did not significantly change H/M ratios and washout rate compared to the standard supine position. Nevertheless, the inferior to anterior uptake ratio on prone imaging increased in early images whereas in late imaging there was no difference compared to supine acquisition. In our study, 10 patients had a BMI ≥30 kg/m2. Noteworthy, obese patients had significantly reduced early and late 123I-MIBG H/M ratios both in supine and prone positions compared to non-obese subjects. These results are in agreement with those of a previous investigation demonstrating that mean H/M ratio progressively decreased with increasing BMI (18). However, also in obese subjects there were no significant differences in early and late H/M ratios and in washout rate between supine- and prone-position acquisitions. These findings suggest that the derangement of sympathetic innervation observed in obese patients with HF is not related to technical reasons. However, the relatively small number of patients of the present study makes our findings preliminary and warranting further confirmation.
Conclusions
Our results indicate that in HF patients, obesity has a significant impact on 123I-MIBG indexes of cardiac sympathetic innervation. Prone-position acquisition did not change early and late123I-MIBG H/M ratios and washout rate compared to supine-position both in obese and non-obese patients. Thus, prone data acquisition may not be considered a valid alternative approach of the standard method to evaluate cardiac innervation using 123I-MIBG planar imaging in obese subjects.
Acknowledgements
None.
Footnote
Conflicts of Interest: Paper accepted for presentation during the Annual Congress of the European Association of Nuclear Medicine (EANM), Hamburg, 10-14 October 2015.
References
- Travin MI. Cardiac autonomic imaging with SPECT tracers. J Nucl Cardiol 2013;20:128-43. [PubMed]
- Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J. ADMIRE-HF Investigators. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212-21. [PubMed]
- Verberne HJ, Somsen GA, Povinec P, van Eck-Smit BL, Jacobson AF. Impact of mediastinal, liver and lung (123)I-metaiodobenzylguanidine ((123)I-MIBG) washout on calculated (123)I-MIBG myocardial washout. Eur J Nucl Med Mol Imaging 2009;36:1322-8. [PubMed]
- Matsunari I, Aoki H, Nomura Y, Takeda N, Chen WP, Taki J, Nakajima K, Nekolla SG, Kinuya S, Kajinami K. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging 2010;3:595-603. [PubMed]
- Yoshinaga K, Tomiyama Y, Manabe O, Kasai K, Katoh C, Magota K, Suzuki E, Nishijima K, Kuge Y, Ito YM, Tamaki N. Prone-position acquisition of myocardial (123)I-metaiodobenzylguanidine (MIBG) SPECT reveals regional uptake similar to that found using (11)C-hydroxyephedrine PET/CT. Ann Nucl Med 2014;28:761-9. [PubMed]
- Petretta M, Bonaduce D, de Filippo E, Mureddu GF, Scalfi L, Marciano F, Bianchi V, Salemme L, de Simone G, Contaldo F. Assessment of cardiac autonomic control by heart period variability in patients with early-onset familial obesity. Eur J Clin Invest 1995;25:826-32. [PubMed]
- Lambert EA, Straznicky NE, Lambert GW. A sympathetic view of human obesity. Clin Auton Res 2013;23:9-14. [PubMed]
- Lambert EA, Straznicky NE, Dixon JB, Lambert GW. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol 2015;309:H244-58. [PubMed]
- Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity (Silver Spring) 2009;17:1232-9. [PubMed]
- Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schäfers M, Somsen GA, Unlu M, Verberne HJ. EANM Cardiovascular Committee. European Council of Nuclear Cardiology. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging 2010;37:1802-12. [PubMed]
- Pellegrino T, Petretta M, De Luca S, Paolillo S, Boemio A, Carotenuto R, Petretta MP, di Nuzzo C, Perrone-Filardi P, Cuocolo A. Observer reproducibility of results from a low-dose 123I-metaiodobenzylguanidine cardiac imaging protocol in patients with heart failure. Eur J Nucl Med Mol Imaging 2013;40:1549-57. [PubMed]
- Bischof Delaloye A, Carrió I, Cuocolo A, Knapp W, Gourtsoyiannis N, McCall I, Reiser M, Silberman B. White paper of the European Association of Nuclear Medicine (EANM) and the European Society of Radiology (ESR) on multimodality imaging. Eur J Nucl Med Mol Imaging 2007;34:1147-51. [PubMed]
- Rispler S, Frenkel A, Kuptzov E, Brodov Y, Israel O, Keidar Z. Quantitative 123I-MIBG SPECT/CT assessment of cardiac sympathetic innervation--a new diagnostic tool for heart failure. Int J Cardiol 2013;168:1556-8. [PubMed]
- Carrió I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc Imaging 2010;3:92-100. [PubMed]
- Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter defibrillator patients. J Am Coll Cardiol 2010;55:2769-77. [PubMed]
- Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parízek P, Agostini D, Knuuti J, Flotats A, Arrighi J, Muxi A, Alibelli MJ, Banerjee G, Jacobson AF. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: a prospective multicenter pilot study. Circ Cardiovasc Imaging 2008;1:131-40. [PubMed]
- Sasano T, Abraham MR, Chang KC, Ashikaga H, Mills KJ, Holt DP, Hilton J, Nekolla SG, Dong J, Lardo AC, Halperin H, Dannals RF, Marbán E, Bengel FM. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol 2008;51:2266-75. [PubMed]
- Jacobson A. Interaction of body mass index and cardiac uptake of 123I MIBG on mortality risk in heart failure subjects. J Nucl Med 2013;54:237.


