Carbon coated superparamagnetic iron oxide nanoparticles for sentinel lymph nodes mapping
Abstract
Intra-operative lymphatic mapping and sentinel lymph-adenectomy (LM/SL) maps the lymphatic path from the primary tumor to the regional nodes and permits selective excision of the first sentinel lymph nodes. It is a well established technique to detect occult regional node metastases for melanoma patients and breast cancer patients. In continuing attempts to improve accuracy, most surgeons now combine a dye (such as carbon particles) and radiopharmaceuticals when performing LM/SL. We developed a proto-type of carbon coated superparamagnetic iron oxide nanoparticles (SPIO@C) for sentinel lymph nodes mapping. Compared with combining carbon particles and radiopharmaceuticals for performing LM/SL, there are a number of advantages with our approach: I. SPIO is an MRI contrast agent, thus pre-operative MRI may be used for LM/SL instead of gamma camera. There is no radiation associated with MRI, and MRI offers good tissue contrast and detailed cross-sectional images. II. There will be only needed one injection of SPIO@C nanoparticles, instead of administrating two successive injections of radiopharmaceuticals and carbon particles. III. During the operation, an intraoperative MRI scanner can be used, or more conveniently by a simple magnetometer.
Keywords
Regional lymph nodes; sentinel lymph node; carbon; MRI; superparamagnetic iron oxide
The spread of some forms of cancer follows an orderly progression, spreading first to regional lymph nodes, then the next echelon of lymph nodes, and so on, since the flow of lymph is directional, i.e. some cancers spread in a predictable fashion from where the cancer started. In these cases, if the cancer spreads it will spread first to lymph nodes close to the tumor before it spreads to other parts of the body. Sentinel lymph node surgery is to see if the cancer has spread to the very first lymph node (the "sentinel lymph node", SLN). If the SLN does not contain cancer, then there is a high likelihood that the cancer has not spread to any other area of the body. Lymph node dissection during surgery with microscopic evaluation is the most accurate method to determine lymph node metastasis.
Detection of occult regional node metastases commonly involves intra-operative lymphatic mapping and sentinel lymphadenectomy (LM/SL). LM/SL technique maps the lymphatic path from the primary tumor to the regional nodes and permits selective excision of the first SLNs. Typically, carbon particles, or a blue dye such as isosulfan, is injected around the primary tumour and travels along the lymphatic vessels. The first lymph nodes to receive lymphatic drainage from the primary tumour are stained dark (carbon color) or blue (for isosulfan), and are harvested as SLN for histology assessment. Particles can pass through the lymphatic vessels but not the blood capillaries mainly due to the difference in permeability. Histology techniques of multilevel sectioning and immunohistochemistry enable the detection of small nodal tumour infiltrates in the SLNs. Directing the pathologist’s attention to a region of the SLNs that is most likely to harbour metastatic deposits may increase the detection rate of small nodal tumour infiltrates (1). Patients with tumor-positive SLNs require complete lymph node dissection, while those without SLN metastases avoid the complications and costs associated with the complete lymph node dissection (2).
LM/SL is now well established for melanoma patients and for breast cancer patients (3-5). The technique is also being applied to a variety of other cancers, including colon cancer and nonsmall cell lung cancer (6,7). Isosulfan blue dye was a commonly used agent to identify the SLNs. However, the relatively rapid washout of dye from the SLN to successive nodes in the basin can lead to intraoperative misidentification of the sentinel node. Morton et al. introduced the use of carbon particles (carbon dye) for LM/SL (5). In medicine, carbon dye has served as both a diagnostic and a therapeutic agent for a long history. For example, carbon dye is used to mark polypectomy sites within the colon to facilitate endoscopic surveillance (8,9). Several studies have documented the safety of carbon dye in clinical use. Maruyama et al. reported no apparent adverse effects of intraperitoneal injection of carbon particle suspensions in more than 3700 patients (10). When used for lymphatic mapping, carbon particles are transported with the lymph from the site of injection to draining lymph nodes where they are phagocyted by macrophages in the subcapsular sinus, leading to a long-term, microscopically detectable mark on the lymph node. This is in contrast to the only temporary blue staining by isosulfan blue. The site of carbon particles inside a tumour-positive SLN can be correlated with the location of nodal tumour infiltrates, therefore allowing ‘‘intranodal mapping’’. SLNs are more likely to contain carbon dye as well as nodal tumour infiltrates (7). Isolated tumour cells are frequently located in the same nodal region as phagocyted carbon particles (7). Hence, LM/SL using carbon particles supports the pathologist by marking the region inside a lymph node most likely to harbour tumour deposits further increases chances of detecting small nodal infiltrates (2,7).
In continuing attempts to improve accuracy, most surgeons now combine a dye (such as carbon particles) and radiopharmaceuticals when performing LM/SL (2). Preoperative lymphoscintigraphy is performed with a colloid agent and on the day of surgery. During surgery, the hand-held gamma probe directs the surgeon to the site of the radioactive (and dye -stained) SLNs (2). For lymphoscintigraphy, radiopharmaceuticals injections are repeated every 20 min if required. Without gamma lymphoscintigraphy, carbon/blue stained afferent lymphatics and nodes can be difficult to find, because it is technically challenging, in a blood-stained surgical operative field, to identify lymph nodes and draining lymphatic vessels hidden in the adjacent fat. Radiopharmaceuticals alone can also be misleading in determining whether a node is sentinel (2). There is still no clear definition of a SLN when radionucleotide tracer techniques are used, and the strong signal from radioisotope injected around the tumor, so-called “shinethrough,” interferes with detection of radioactivity from lymph nodes (11,12). Radiopharmaceuticals can cause radiation hazard not only to the patients, also to the health care staff. The logistics of injecting radiopharmaceuticals in the operating room present difficulties as well.
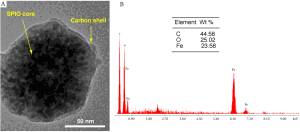
We developed a proto-type of carbon coated superparamagnetic iron oxide nanoparticles (SPIO@C) for SLN mapping. Monodisperse SPIO nanoparticles was synthesized by a modified solvothermal method using FeCl3•6H2O as precursor and ethylene glycol (EG)/ diethylene glycol (DEG) as solvent. The reaction was carried out in a sealed autoclave at high temperature. The particle size can be tuned (30-200 nm) by varying the EG/ DEG ratios and reaction time. To synthesize the SPIO@C core/ shell nanoparticles, the SPIO (Fe3O4) nanoparticles were first redispersed in glucose aqueous solution and then transferred to autoclaves and heated at high temperature. A carbonization process of the glucose was facilitated onto the surface of Fe3O4 nanoparticles under the hydrothermal conditions. The thickness (10-100 nm) of the carbon shell can be tuned by changing the reaction time. With our proto-type SPIO@C, the overall size is similar to those carbon particles currently in clinical use (100-150 nm) (Figure 1).
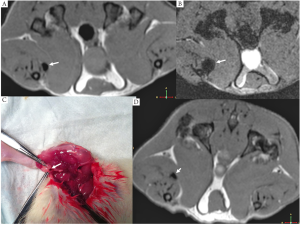
In a pilot study, SPIO@C suspension was injected to the right foot pad of two rats intradermally. MRI of the lower limb was performed 2 hour later, then the right lower limb was dissected and dark stained (carbon stained) lymph nodes were indentified. MRI demonstrated SPIO induced signal void for lymph nodes, with gradient echo images demonstrating larger signal void area than the turbo spin echo images (Figure 2).
Compared with combining carbon particles and radiopharmaceuticals for performing LM/SL (2), there will be a number of advantages with our approach: I. SPIO is an MRI contrast agent (13,14), thus pre-operative MRI may be used for LM/SL instead of gamma camera. There is no radiation associated with MRI, and MRI offer good tissue contrast and detailed cross-sectional images. II. There will be only needed one injection of SPIO@C nanoparticles, instead of administrating two successive injections of radiopharmaceuticals and carbon particles. III. During the operation, an intra-operative MRI scanner can be used, or more conveniently by a simple magnetometer (15). IV. The present used carbon nanoparticles cannot be dispersed well in water, instead, they can only be injected by mixing with a surfactant. Our proposed SPIO@C nanoparticles will have good water dispersibility.
Further optimization and validation studies are being carried out in our laboratories.
References
- Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg 1995;222:394-9; discussion 399-401.[LinkOut]
- Cochran AJ, Essner R, Rose DM, et al. Principles of sentinel lymph node identification: background and clinical implications. Langenbecks Arch Surg 2000;385:252-60.[LinkOut]
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9.[LinkOut]
- Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;220:391-8; discussion 398-401.[LinkOut]
- Morton DL, Hoon DS, Cochran AJ, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg 2003;238:538-49; discussion 549-50.[LinkOut]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92.[LinkOut]
- Viehl CT, Guller U, Hamel CT, et al. Carbon dye staining of sentinel lymph nodes facilitates microstaging of colon cancer patients. World J Surg 2006;30:453-6.[LinkOut]
- Ponsky JL, King JF. Endoscopic marking of colonic lesions. Gastrointest Endosc 1975;22:42-3.[LinkOut]
- Botoman VA, Pietro M, Thirlby RC. Localization of colonic lesions with endoscopic tattoo.Dis Colon Rectum 1994;37:775-6.[LinkOut]
- Maruyama K, Sasako M, Kinoshita T, et al. [Reasonable lymph node dissection in radical gastrectomy for gastric cancer: introduction of computer information system and lymphography technique by India-ink]. Nihon Geka Gakkai Zasshi 1989;90:1318-21.[LinkOut]
- Ueda K, Suga K, Kaneda Y, et al. Radioisotope lymph node mapping in nonsmall cell lung cancer: can it be applicable for sentinel node biopsy? Ann Thorac Surg 2004;77:426-30.[LinkOut]
- Liptay MJ, Masters GA, Winchester DJ, et al. Intraoperative radioisotope sentinel lymph node mapping in non-small cell lung cancer. Ann Thorac Surg 2000;70:384-9; discussion 389-90.[LinkOut]
- Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 2001;11:2319-31.[LinkOut]
- Wang YX. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg 2011;1:35-40.[LinkOut]
- Minamiya Y, Ito M, Katayose Y, et al. Intraoperative sentinel lymph node mapping using a new sterilizable magnetometer in patients with nonsmall cell lung cancer. Ann Thorac Surg 2006;81:327-30.[LinkOut]


