Image-guided percutaneous microwave ablation of small renal tumours: short- and mid-term outcomes
Introduction
Renal cancer is the third most common urologic cancer after prostate and bladder cancers (1). It is the sixth leading cause of death per solid cancer in industrialized countries (2). The growing use of imaging studies [computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US)] in order to access many diseases has profoundly changed the natural history of kidney cancer. Currently, almost half of kidney tumours are discovered fortuitously (3). The evolution of imaging techniques enables the detection of small renal tumours, often asymptomatic and non metastatic, therefore associated with better prognosis. The different presentation and prognosis of these small renal cancers have encouraged the development of nephron-sparing treatments. Thus, partial nephrectomy has become the standard treatment for localized tumours stage T1 (≤7 cm) (4). However, many patients with small renal cell carcinoma (RCC) are not eligible for surgical treatment due to several reasons: multiple comorbidities, advanced age and already altered renal function.
New percutaneous ablative treatments have therefore emerged. Thermal ablation by radiofrequency or cryotherapy is increasingly considered as a reliable alternative to treat inoperable patients (4). Other percutaneous ablative techniques, such as microwave ablation (MWA) were developed more recently. MWA has several theoretical advantages over other available techniques (5,6): higher and homogeneous heating temperature, faster procedure, greater volume of tumour ablation and more complete destruction of tumours close to blood vessels due to reduced cooling. For these reasons, in our practice, the majority of percutaneous renal tumours destruction is now performed by MWA. Nevertheless, there are only few series currently available in the literature reporting the efficiency of this technique.
The aim of our study was to evaluate the efficacy, complications and mid-term results of MWA for inoperable patients with small renal tumours. Given that MWA was a common practice, the local ethics committee did not request additional consent.
Materials and methods
Patients
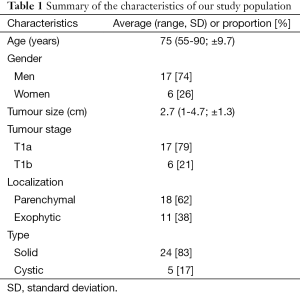
Between August 2012 and February 2015, 29 tumours were treated with MWA in 23 patients in our academic hospital: 20 patients with a single tumour targeted, one patient with two tumours targeted, one patient with three tumours targeted and one patient with four tumours targeted. Characteristics of our study population are summarized in Table 1. Patients included were all referred by urologists after being disqualified from surgical treatment for different reasons: advanced age ≥75 years (14 patients), significant comorbidities (14 patients with synchronous cancer in another organ during treatment), bilateral or multiple tumours (three patients), hereditary cancer (two cases of Von Hippel Lindau disease), recurrence after partial nephrectomy (one patient), recurrence on solitary kidney after contralateral total nephrectomy (one patient) or kidney transplant (one patient).
Full table
For patients to be eligible for this treatment, the targeted tumour had to be in an accessible location of tumour stage T1 (≤7 cm) without any lymph node involvement (N0) or distant metastasis (M0). The choice of this treatment was validated in a multidisciplinary meeting.
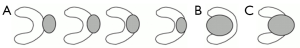
The depth of the tumour was defined according to four stages depending on its relations with perirenal fat, renal parenchyma and hilar structures (vessels, excretory cavities) (Figure 1) (7). Only exophytic (n=18) and parenchymal (n=11) tumours were treated in our series. No central or mixed tumours were treated.
Of 23 patients, four were on dialysis for chronic terminal kidney disease (CKD), seven were moderate CKD [glomerular filtration rate (GFR) estimated between 30-60 mL/min].
Only eight patients in our series underwent a biopsy prior to the ablathermy, that confirmed the diagnosis of RCC in all cases. The other patients had either typical imaging findings with cystic lesions characterised as Bosniak IV or a marked evolution between two successive examinations.
MWA procedure
In all cases the same system was used: MTA Aculis (AngioDynamics, NY, USA) which consists of a microwave generator, a control station (temperature monitoring during the ablation) and applicators or antennas with integrated cooling system. The generator delivers a power of 60 to 140 W at an operating frequency of 2.45 GHz. Only one applicator can be used at a time, available in three lengths, 14, 19 or 29 cm, for a diameter of 15 Gauges or 1.8 mm. A pump system provides a flow of saline in the cable and within the applicator, in order to prevent overheating of the applicator in normal tissues.
An applicator per patient was used at a cost of €1,385 excluding tax. Each patient was seen during a consultation by the radiologist prior to performing the gesture, to explain the procedure, possible complications and collect oral and written consent.
Hospitalization was scheduled the day before the treatment in the urology department for 2 days (discharge the day after the treatment in the absence of complications). Ablathermy procedures were all carried out under general anaesthesia. The applicator was positioned percutaneously under CT or US guidance. The position of the applicator was controlled by a CT acquisition before heating. The ablation volume and ablathermy duration were defined prior to the procedure according to the manufacturer’s instructions.
A hydrodissection technique was used in five patients with tumours close to the colon to protect it. A CT scan was performed immediately after thermal ablation to access the volume of tumour destruction and the absence of immediate complications (hematoma, urinoma) (Figure 2). All procedures were performed by two experienced interventional radiologists (R Loffroy and JP Cercueil, with 17 and 10 years of experience, respectively).
Follow-up
Each patient was monitored in the urology department after treatment: vital signs were taken, pain was assessed with a visual analog scale, blood samples were drawn to include the analysis of serum creatinine levels.
A follow-up with renal MRI was performed in all patients at 2 months, 6 months, 1 year and annually for 5 years when no recurrence was detected, in addition to the usual clinical oncology follow-up by the urologist in charge of the patient. Only one patient was followed by CT of the abdomen and pelvis with contrast medium injection, because of a pacemaker placement 6 months after thermal ablation. The MRI protocol was standardized and included Haste axial and coronal T2-weighted fast sequences, diffusion sequences and especially fat-sat turbo spin echo (TSE) T1-weighted sequences without and with intravenous injection of gadolinium with contrast-enhanced dynamic study and subtractions.
Definition
The primary endpoint of this study was the technical efficiency, defined by the absence of residual vascularized tumour on MRI control performed 2 months after treatment. Any residual enhancement confirmed by subtraction sequences was considered as residual tumour and thus as failure of the procedure. Clinical success corresponded to the absence of tumour recurrence at the following MRI beyond 2 months. Complications were classified as minor or major according to the Society of Interventional Radiology (SIR).
Statistical analysis
The quantitative values (patient age, tumour size, length of hospital stay and follow-up time) were expressed as mean, standard deviation (SD) and the inter-quartile range (IQR). Qualitative values (technical efficiency, clinical efficacy, mortality, complications) were expressed by frequency and proportion. Differences in creatinine levels were analyzed using the Student paired t-test or using the paired Wilcoxon test. The significance level adopted was P<0.05. Length of survival without recurrence was calculated using Kaplan-Meier method.
Results
The MWA procedure was performed in 23 patients with a technical efficiency of 96%, only one patient did not benefit from the MRI control at 2 months (death after the procedure). One ablathermy procedure by tumour was sufficient for all patients. The procedure was rapid in most patients as a single heating of the tumour was necessary (duration ≤6 min per tumour).
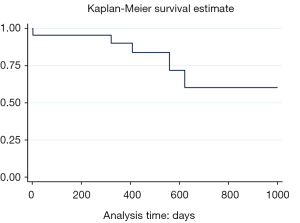
During a mean follow-up of 12.2 months (IQR: 0-25 months, SD: 6.6 months), no locoregional or distant recurrence were observed, indicating a clinical success rate of 100%. The recurrence-free survival rate was 78% (Figure 3). The average length of hospitalization was 2.2 days (IQR: 1-5 days, SD: 1 day).
One patient experienced a major complication (4%). He experienced a hypertensive crisis in the immediate course of the procedure leading to an acute pulmonary oedema complicated by cardiogenic shock and death. Three minor complications (13%) were identified: a perirenal hematoma, a perirenal urinoma and a fracture of the tip of the applicator (1 cm to the tip) after repositioning in a patient with several targeted tumours. No additional specific treatment was required for these complications. Among the 13 of 23 patients with available data included in the statistical analysis, no significant changes in renal function (creatinine levels) were noted before treatment and at day 1 after treatment (P=0.57).
Discussion
The therapeutic options for small localized renal tumours are surgery (partial or total nephrectomy), thermal ablation or active surveillance (4). Partial nephrectomy is considered the gold standard treatment. It remains a risky surgery with significant complication rates. Some authors have reported up to 20% of perioperative complications including 6% of patients requiring revision surgery, even if complications are less frequent with new robot-assisted surgical techniques (8). Also, some kidney tumours occur in locations not as easily accessible to surgery.
Increasingly, kidney tumours are discovered incidentally at a localized stage in elderly patients, with significant comorbidities, severe renal impairment, short life expectancy or a major surgical risk. In these patients at risk for surgery, thermal ablative techniques as radiofrequency ablation or cryotherapy are recognized today as reliable therapeutic alternatives. Many studies have shown that technical efficiency and recurrence-free survival rates were comparable between partial nephrectomy and thermal ablation techniques (9,10). Furthermore, percutaneous techniques allow preservation of renal function (11), which is essential given the pejorative consequences of chronic kidney disease in terms of morbidity and mortality. The MWA technique has several theoretical advantages and recent publications found very encouraging results in this indication (12-16). Yu et al. (14) reported a comparable oncological effectiveness between US-guided MWA (65 patients) and total nephrectomy (98 patients) on a series of 163 patients after a 5-year follow-up. Lin et al. (15) reported a technical effectiveness of 93.8% of the MWA without major complications for the treatment of RCC in a series of 14 patients with solitary kidney. Carrafiello et al. (16) reported a 100% efficiency rate of the MWA technique without major complications for the treatment of seven cystic lesions Bosniak III or IV. The MWA technique may also be used safely in patients with an implantable cardiac device because of the absence of dispersion plate (17), such as for radiofrequency.
Our study, one of the most important to date, corroborates the literature results with a technical efficiency of 96% and shows that the MWA is an effective and safe technique for the treatment of localized small renal tumours. Our results in terms of survival are more disappointing, since four patients died during follow-up. However, none of the four deaths were directly attributable to local recurrence or metastatic renal tumours or ablathermy: one death from cardiogenic shock complicating a heart attack 18 months after the procedure, and three deaths related to another metastatic cancer (pleural, prostate and pancreas primary cancer, respectively). Moreover, there is clearly a bias selection since all of our patients were challenged for surgical treatment. Most had significant comorbidities including 14 patients with other cancer during treatment. These patients had from the start a short life expectancy lowering the sample survival. Only one major complication occurred shortly after thermal ablation procedure and caused the death of the patient 2 days after the procedure due to acute pulmonary oedema. However, this complication was due to an important but underestimated anaesthetic risk in a fragile patient of 85 years.
All MWA procedures were performed under CT guidance or in combination with US guidance. Some teams work under US guidance alone. Nevertheless, we believe that the CT guidance has several advantages: more accurate determination of tumour limits and relations of tumours to treat, more accurate positioning of the applicator, control of thermal ablation zone after heating, possibility to treat more targeted lesions on the same kidney without significant motion artifact, reliable detection of early complications such as hematoma or urinoma. The US guidance can be as effective, fast and accurate to position the applicators especially for superficial lesions, in case of respiratory motion. However, US guidance is limited by the narrow acoustic window and formation of gas bubble altering the image quality with artifacts after starting of the heating.
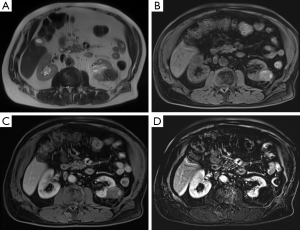
All patients were monitored by renal MRI at 2 months, 6 months, 1 year and annually in the absence of recurrence. Many authors have described the changes in appearance over time of a tumour after thermal ablation (18,19). There is currently no published study comparing the differences in appearance after microwave treatment and other methods of thermal ablation. The most sensitive imaging techniques for monitoring are CT scan with contrast medium injection and especially renal MRI with subtraction sequences, which is more sensitive for the detection of a residual tumour contrast enhancement (Figure 4). Moreover, the injection of contrast media is crucial to detect a residual tumour or a recurrence. Macrocyclic gadolinium products can be injected to limit the risks of nephrogenic systemic fibrosis in patients with poor renal function. MRI is therefore the method of choice in our practice for these reasons and for radiations purposes, despite its higher cost and lower availability.
Our study has several limitations, in particular because of the retrospective data collection. Due to limited number of patients, our study lacks power and cannot highlight differences compared to the other thermal ablative techniques currently available. A longer-term assessment is also necessary to draw any reliable conclusions in terms of oncologic efficacy. Also, all Cancer Committees of Urological Associations recommend that a biopsy is performed for histological evidence of malignancy before thermal ablation procedure (grade C) (4). Only eight patients in our series underwent a biopsy to confirm the diagnosis of RCC. We did not biopsy five cystic masses because the Bosniak classification allowed us to reliably assess the risk of malignancy (type IV). The other 11 patients all had a typical imaging progression of the lesion between two successive examinations. Conducting a biopsy during an additional procedure prior to ablation was debatable in these patients with a short life expectancy, especially as most of them were under antiplatelet therapy and/or anticoagulant. Performing a biopsy during the same session as the ablation did not seem very useful and therefore not ethically justified. Indeed, histologic diagnosis in this case will have no impact on the patient management since the ablation will already be performed by the time the results would be received. Even if the biopsy results were negative, it would be more careful not to reduce follow-up. Indeed, the risk of false negative biopsy remains significant (1%) (20). In addition, some authors have shown that there may be recurrence in these patients. Permpongkosol et al. (21) for example, have found two cases of recurrence after thermal ablation in 19 patients with prior negative biopsy (21). Moreover, it is logical not to biopsy cystic lesions. Finally, it seems important to note that our results are valid with the MTA Aculis system. There are indeed many differences between MWA devices currently available, making future comparison with different devices and needles necessary.
Conclusions
The MWA technique appeared to be an effective therapeutic alternative, safe and fast for the treatment of localized small exophytic and parenchymal renal tumours in non-operable patients. It would be necessary to conduct a randomized prospective study comparing MWA to surgical treatment and to other percutaneous ablation techniques to define the future role of this specific therapy in the management of selected patients with localized RCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Buy X, Lang H, Garnon J, Gangi A. Percutaneous ablation of renal tumors: radiofrequency ablation or cryoablation? J Radiol 2011;92:774-88. [PubMed]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51:203-5. [PubMed]
- Patard JJ, Baumert H, Bensalah K, Bernhard JC, Bigot P, Escudier B, Grenier N, Hétet JF, Long JA, Méjean A, Paparel P, Richard S, Rioux-Leclercq N, Coloby P, Soulié M. Les membres du CCAFU. CCAFU Recommendations 2013: Renal cancer. Prog Urol 2013;23 Suppl 2:S177-204. [PubMed]
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 2010;38:65-78. [PubMed]
- Floridi C, De Bernardi I, Fontana F, Muollo A, Ierardi AM, Agostini A, Fonio P, Squillaci E, Brunese L, Fugazzola C, Carrafiello G. Microwave ablation of renal tumors: state of the art and development trends. Radiol Med 2014;119:533-40. [PubMed]
- Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology 2000;217:665-72. [PubMed]
- Lowrance WT, Yee DS, Savage C, Cronin AM, O'Brien MF, Donat SM, Vickers A, Russo P. Complications after radical and partial nephrectomy as a function of age. J Urol 2010;183:1725-30. [PubMed]
- Katsanos K, Mailli L, Krokidis M, McGrath A, Sabharwal T, Adam A. Systematic review and meta-analysis of thermal ablation versus surgical nephrectomy for small renal tumours. Cardiovasc Intervent Radiol 2014;37:427-37. [PubMed]
- Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, Psutka SP, Stewart SB, Callstrom MR, Cheville JC, Boorjian SA, Leibovich BC. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015;67:252-9. [PubMed]
- Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 2008;179:75-9; discussion 79-80. [PubMed]
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology 2011;77:792-7. [PubMed]
- Carrafiello G, Mangini M, Fontana F, Recaldini C, Piacentino F, Pellegrino C, Laganà D, Cuffari S, Marconi A, Fugazzola C. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol 2010;33:367-74. [PubMed]
- Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Zhang X, Dong J, Mu MJ, Li X, Wang XH. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology 2014;270:880-7. [PubMed]
- Lin Y, Liang P, Yu XL, Yu J, Cheng ZG, Han ZY, Liu FY. Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology 2014;83:357-63. [PubMed]
- Carrafiello G, Dionigi G, Ierardi AM, Petrillo M, Fontana F, Floridi C, Boni L, Rovera F, Rausei S, Mangano A, Spampatti S, Marconi A, Carcano G, Dionigi R. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg 2013;11 Suppl 1:S30-5. [PubMed]
- Skonieczki BD, Wells C, Wasser EJ, Dupuy DE. Radiofrequency and microwave tumor ablation in patients with implanted cardiac devices: is it safe? Eur J Radiol 2011;79:343-6. [PubMed]
- Wile GE, Leyendecker JR, Krehbiel KA, Dyer RB, Zagoria RJ. CT and MR imaging after imaging-guided thermal ablation of renal neoplasms. Radiographics 2007;27:325-39; discussion 339-40. [PubMed]
- Iannuccilli JD, Grand DJ, Dupuy DE, Mayo-Smith WW. Percutaneous ablation for small renal masses-imaging follow-up. Semin Intervent Radiol 2014;31:50-63. [PubMed]
- Schmidbauer J, Remzi M, Memarsadeghi M, Haitel A, Klingler HC, Katzenbeisser D, Wiener H, Marberger M. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 2008;53:1003-11. [PubMed]
- Permpongkosol S, Link RE, Solomon SB, Kavoussi LR. Results of computerized tomography guided percutaneous ablation of renal masses with nondiagnostic pre-ablation pathological findings. J Urol 2006;176:463-7; discussion 467. [PubMed]