
This article has an erratum available at: http://dx.doi.org/10.21037/qims-2021-06 the article has been update on 2021-11-19 at here.
Clinical presentation and imaging characteristics of clear cell sarcoma-like tumour of the gastrointestinal tract with liver metastasis: a case description
Introduction
The clear cell sarcoma-like tumor of the gastrointestinal tract (CCSLTGT) is an unusual malignant soft tissue tumor in the gastrointestinal tract. Although initially identified as a type of clear cell sarcoma (CCS) (1), further studies revealed it to be a new entity that resembles but is different from CCS (2).
Only 96 cases have been reported worldwide (3), mostly occurring in the small intestine. Only 13 cases have been reported in the stomach (2-7). However, few studies have been conducted to evaluate the imaging characteristics of CCSLTGT. We report an extremely rare case of CCSLTGT of the stomach with liver metastasis in a middle-aged woman.
Case presentation
All procedures performed in studies involving human participants followed the ethical standards of the institutional and/or national research committee(s) and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
A 49-year-old woman presented with recurring intermittent melena for half a month without obvious inducement. The patient experienced gradually progressive dizziness, fatigue, and syncope.
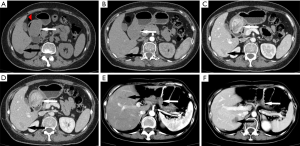
The axial image of the upper abdominal plain computed tomography (CT) scan revealed an irregular soft tissue mass, approximately 42 mm × 35 mm in size, protruding into the gastric cavity [30–44 Hounsfield units (HU)], with areas of lower density (12 HU). Additionally, a dot-like hyperdense calcification was detected inside the mass (128 HU). The radiodensity at the same level of musculature was approximately 33 HU on the plain CT scan, and the ratio of the mean CT value of the mass to the CT value of the muscles was 1.12 (Figure 1A). A contrast-enhanced scan in the arterial phase showed a mild enhancement of the mass with uneven levels of radiodensity (57 HU in the solid parts and 37 HU in other parts). In the arterial phase, the radiodensity at the same level of musculature was 48 HU, and the ratio increased to 1.19 (Figure 1B). In the venous phase, distinct heterogeneous enhancement of the mass in the gastric antrum was observed, reaching nearly 182 HU. The lower-density areas were also enhanced to a lesser degree, reaching nearly 56 HU. The radiodensity at the same level of musculature was 60 HU, and the ratio significantly increased to 3.03 (Figure 1C). In the delayed phase, the uneven enhancement remained; however, the radiodensity levels were lower than in the venous phase. The radiodensity of the gastric antrum lowered to approximately 144 HU, and the irregular, saccate, lower density area was approximately 55 HU. The radiodensity at the same level of musculature was 58 HU (Figure 1D). In the right posterior lobe of the liver, the plain CT scan revealed a hypodense shadow approximately 32 mm in size and with a radiodensity of approximately 27 to 57 HU, with a relatively unclear edge. In addition, the normal liver parenchyma was 65 HU. The lesion was slightly unevenly enhanced in the arterial phase, ranging from 43 to 89 HU. The venous phase image of the liver revealed that the density of the lesion was increasingly enhanced (72 to 155 HU). This enhancement continued in the delayed phase (88 to 157 HU) (Figure 1A,B,C,D).
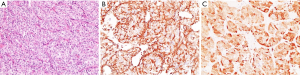
A surgical biopsy of the wall of the gastric antrum showed that the tumor was composed of epithelioid cells arranged in sheets and nests with local ulceration. Immunohistochemistry showed strong, positive expression of the focal nerve or neuroectodermal markers vimentin (+) and CD56 (+) and diffuse S100 (Figure 2), coupled with a complete absence of melanocytic markers (HMB45 and Melan-A) (7,8). The pathology of the tumor and the liver metastasis were consistent with CCSLTGT.
The patient underwent laparoscopic distal gastrectomy and liver tumor resection. She refused to receive any adjuvant therapy. Tenth months after the operation, the tumor recurred and was accompanied by liver metastases.
The follow-up CT scan ten months post-surgery showed changes in the stomach and liver. The stomach wall near the surgical margin had thickened, and there were multiple rounds, slightly low-density shadows of varying sizes in the liver (Figure 1E,F).
The second pathological manifestation of the masses, combined with immunohistochemistry, was consistent with CCSLTGT with lymph node metastases.
Discussion
CCSLTGT is most common in young adults (median age 33 years), with no sex predilection (9). Most patients present with symptoms such as abdominal pain, weight loss, fatigue, and nausea. The median CCSLTGT diameter is 45 mm. In our case, the patient was 49 years old and presented with a 42 mm tumor in her stomach, which shares the features of CCSLTGT cases reported in previous studies. These tumors often grow large before detection and are accompanied by hemorrhage, necrosis, or cystic changes. CCSLTGT grows slowly but is highly aggressive and prone to metastasis, leading to a poor prognosis and a low survival rate (1-3,7-11). Due to the lack of specific clinical manifestations and the limitations of gastrointestinal endoscopy, diagnosis is difficult. Therefore, CT is particularly important to confirm a diagnosis of CCSLTGT. Our patient underwent a CT scan to observe and analyze the manifestations of the gastric tumors and liver metastasis. CT has a fast scanning speed and high resolution, making it a convenient tool for early diagnosis. Moreover, it can provide more information than other methods, such as the exact depth of the tumor and the fat space around the gastrointestinal tract.
However, as CCSLTGT is extremely rare, there have been fewer than 100 reported cases, and the reports have focused mainly on pathological features and treatment. Thus, there are no thorough radiographic descriptions, let alone a case report of CCSLTGT of the stomach with liver metastasis with imaging details (7,11). Therefore, we herein report a case of CCSLTGT in the stomach combined with liver metastasis and provide detailed imaging characteristics.
We made several notable observations by reviewing the pathological features and the few available CT images of CCSLTGT provided in the literature (7,11,12). The available case reports of intestinal CCSLTGT that vaguely describe imaging characteristics typically describe masses protruding from the cavity (8,9), which is different from CCSLTGT of the stomach. In our case, no lesions protruded from the cavity or broke through the serosa. However, the appearance of the inner part of the cavity in these cases seemed to be identical to the images in our case (3,11). The CT images from the studies in the literature reveal that CCSLTGT masses generally appear on plain CT as unevenly isodense masses that occur in the gastrointestinal tract, with luminal stenosis and uneven enhancement (3,7,11,12). The CCSLTGT in our case was slightly enhanced in the arterial phase, significantly enhanced in the venous phase, and remained enhanced in the delayed phase. We speculate that the tumor had neuroendocrine properties (7,12) that caused characteristic enhancement on contrast CT. In addition, tumor necrosis (6,9) may account for the irregular, small saccate hypodense and low-enhanced shadows in the CT images. Incomplete necrosis within the remaining solid components could have led to slight enhancement in the venous and delayed phases. Moreover, previous case studies reported lymph node metastasis at the time of diagnosis, while no swollen lymph nodes were found in our case (3,6).
There is limited data available regarding systemic therapy and postoperative routine adjuvant therapy in CCSLTGT. Libertini et al. (13) and Li et al. (3) found that aggressive chemotherapy and postoperative routine adjuvant therapy were ineffective in delaying recurrence and prolonging survival, implying that surgery is the recommended treatment. Our patient underwent radical resection and quickly experienced recurrence without any further therapy. This is consistent with previous case studies that report increased possibility of recurrence and distant metastasis in patients who were diagnosed with metastasis at the first presentation (3,8,10). The patient was diagnosed with recrudescent lesions in both the stomach and the liver due to the distinguishing imaging characteristics that were observed at the initial visit. The lesions were also confirmed by pathology. It was unfortunate that the patient in our case refused any adjuvant therapy after recrudescence. Otherwise, we could have studied this case further to guide clinicians and better treatment for those who suffer from CCSLTGT.
CCSLTGT should be differentiated from Malignant gastric Gastrointestinal Stromal Tumors (GIST) and Gastric Cancer (GC). Malignant gastric GIST mostly occurs in the fundus and body of the stomach, with irregular or lobulated shape (14,15) and uneven enhancement in the venous phase in CT (16). Additionally, skip metastasis is readily seen, and liver metastases can show calcification or central areas of necrosis or hemorrhage (17,18). GC is typically located in the antrum, where its invasive growth usually causes the gastric mucosal folds to disappear while the entire stomach wall thickens and hardens with central ulceration in the tumor (18). CT scans reveal progressive and continuous enhancement, but the degree of enhancement is lower than blood vessels. The incidence of omentum infiltration and abdominal implantation is high (19).
In conclusion, CCSLTGT’s CT imaging characteristics of our case are as follows: an irregular mass is detected in the stomach with uneven density and intraluminal growth pattern. The overall tumor shows heterogeneous enhancement in contrast CT (slightly enhanced in the arterial phase, especially significantly enhanced in the venous phase, and remaining enhanced in the delayed phase), with incomplete necrosis changes, liver or lymph node metastasis.
Our study remains limited by the absence of molecular analysis. The molecular analysis aids in the diagnosis of CCSLTGT and will be included in future studies.
We must be alert to the possibility of CCSLTGT when observing significant enhancement to gastrointestinal tumors in the venous phase, especially when accompanied by liver or lymph nodes metastasis. MRI has the unique advantages of high-resolution and functional imaging and can provide an early indication of the disease to prompt an early biopsy when necessary. Currently, the diagnosis of CCSLTGT may still depend on pathological manifestations.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/qims-21-186). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zambrano E, Reyes-Mugica M, Franchi A, Rosai J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol 2003;11:75-81. [Crossref] [PubMed]
- Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, Adsay V, Chou PM, Amanuel B, VanTuinen P, Zambrano EV. Malignant Gastrointestinal Neuroectodermal Tumor: Clinicopathologic, Immunohistochemical, Ultrastructural, and Molecular Analysis of 16 Cases With a Reappraisal of Clear Cell Sarcoma-like Tumors of the Gastrointestinal Tract. Am J Surg Pathol 2012;36:857-68. [Crossref] [PubMed]
- Li R, Cao J, Chen L, Cui F, Chen S, Feng Z, Li N. Malignant Gastrointestinal Neuroectodermal Tumors: Clinicopathological and Prognostic Features of 96 Patients. Onco Targets Ther 2020;13:9731-40. [Crossref] [PubMed]
- Kong J, Li N, Wu S, Guo X, Gu C, Feng Z. Malignant gastrointestinal neuroectodermal tumor: A case report and review of the literature. Oncol Lett 2014;8:2687-90. [Crossref] [PubMed]
- Boland JM, Folpe AL. Oncocytic variant of malignant gastrointestinal neuroectodermal tumor: a potential diagnostic pitfall. Hum Pathol 2016;57:13-6. [Crossref] [PubMed]
- Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, Lu Z, Zhou X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch 2006;448:200-3. [Crossref] [PubMed]
- Chang B, Yu L, Guo W, Sheng W, Wang L, Lao I, Huang D, Bai Q, Wang J. Malignant Gastrointestinal Neuroectodermal Tumor: Clinicopathologic, Immunohistochemical, and Molecular Analysis of 19 Cases. Am J Surg Pathol 2020;44:456-66. [Crossref] [PubMed]
- Askan G, Kombak FE, Seven IE, Basturk O. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract. J Gastrointest Cancer 2019;50:651-6. [Crossref] [PubMed]
- Alyousef MJ, Alratroot JA, ElSharkawy T, Shawarby MA, Al Hamad MA, Hashem TM, Alsayyah A. Malignant gastrointestinal neuroectodermal tumor: a case report and review of the literature. Diagn Pathol 2017;12:29. [Crossref] [PubMed]
- Green C, Spagnolo DV, Robbins PD, Fermoyle S, Wong DD. Clear cell sarcoma of the gastrointestinal tract and malignant gastrointestinal neuroectodermal tumour: distinct or related entities? A review. Pathology 2018;50:490-8. [Crossref] [PubMed]
- Wang Y, Chen T, Lu X, Zhang B. Malignant gastrointestinal neuroectodermal tumor in the small intestine with liver metastasis: First case report worldwide. Asian J Surg 2020;43:769-72. [Crossref] [PubMed]
- Wang J, Thway K. Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med 2015;139:407-12. [Crossref] [PubMed]
- Libertini M, Thway K, Noujaim J, Puls F, Messiou C, Fisher C, Jones RL. Clear Cell Sarcoma-like Tumor of the Gastrointestinal Tract: Clinical Outcome and Pathologic Features of a Molecularly Characterized Tertiary Center Case Series. Anticancer Res 2018;38:1479-83. [PubMed]
- Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Anorectal gastrointestinal stromal tumors: CT and MR imaging features with clinical and pathologic correlation. AJR Am J Roentgenol 2003;180:1607-12. [Crossref] [PubMed]
- Horton KM, Juluru K, Montogomery E, Fishman EK. Computed tomography imaging of gastrointestinal stromal tumors with pathology correlation. J Comput Assist Tomogr 2004;28:811-7. [Crossref] [PubMed]
- Li R, Gan H, Ni S, Fu Y, Zhu H, Peng W. Differentiation of Gastric Schwannoma From Gastric Gastrointestinal Stromal Tumor With Dual-Phase Contrast-Enhanced Computed Tomography. J Comput Assist Tomogr 2019;43:741-6. [Crossref] [PubMed]
- Zhu H, Chen H, Zhang S, Peng W. Differentiation of gastric true leiomyoma from gastric stromal tumor based on biphasic contrast-enhanced computed tomographic findings. J Comput Assist Tomogr 2014;38:228-34. [Crossref] [PubMed]
- Kim HC, Lee JM, Choi SH, Kim KW, Kim SH, Lee JY, Han JK, Choi BI. Imaging of gastrointestinal stromal tumors. J Comput Assist Tomogr 2004;28:596-604. [Crossref] [PubMed]
- Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr 1998;22:288-94. [Crossref] [PubMed]


