
Quantitative and qualitative analysis of fetal temporal lobe T2 signal in cytomegalovirus infected fetuses and normal controls
Introduction
Cytomegalovirus (CMV) is the leading prenatal infection in the developed world (1). In most fetuses, it is asymptomatic. However, in up to 25% of cases, CMV infection has clinical sequela such as neurodevelopmental impairment and sensorineural deafness, significantly impacting life quality (2,3). CMV may infect cell types critical for brain development, like neurons, glia and endothelial cells, resulting in disruption of neuronal proliferation, migration and cortical organization (4-7). Brain pathology following in-utero CMV infection markedly depends on the gestational age at the time of infection. Significant loss of neuronal cells and diffuse disruptions of migration occurs when CMV infection occurs before 16–18 weeks of gestation. Such first or early second trimester infection poses a higher risk for microcephaly, leukoencephalopathy, and polymicrogyria (8,9). Commonly accompanying signs on fetal imaging include ventriculomegaly, calcifications, periventricular pseudocysts, intraventricular synechia, and white matter disease (WMD) (10,11). Later infection, between 18 and 24 weeks of gestation might still result in cortical organization anomalies such as polymicrogyria, whereas third-trimester infection usually has a normal gyral pattern (12-15). Magnetic resonance imaging (MRI) findings are often nonspecific in such later infections, with WMD being the most commonly described CNS anomaly (16).
Investigation of virological markers in amniotic fluid, as CMV fetal viral load, might lack accuracy in detecting fetuses, which will be symptomatic at birth (10). Therefore, detailed and careful prenatal imaging is recommended. A normal prenatal brain assessment by ultrasound and MRI is associated with a good prognosis, whereas abnormal brain imaging is likely to be found in neurologically symptomatic neonates (11,17). Currently, ultrasound is the main imaging modality for screening for prenatal cerebral abnormalities, though fetal brain MRI (fbMRI) as a surrogate imaging modality is increasing. Despite extensive literature, in-utero CMV infection remains relatively underdiagnosed, particularly in cases not associated with characteristic fetal imaging findings.
Fetal ultrasound features related to CMV infection are numerous and nonspecific, as ventriculomegaly, calcifications, or periventricular pseudocysts (18,19). The use of fbMRI in these suspected CMV-infected fetuses reveals subtle cerebral findings, such as white matter (WM) signal changes, particularly in the temporal lobes (20-22). In a meta-analysis recently published, Buca et al. highlighted the potential role of fbMRI, even in fetuses with no anomalies detected on ultrasound, as anomalies can be detected exclusively on MRI in up to 6% of cases (23).
Nevertheless, evaluating these WM signal abnormalities on fbMRI is subjective and based on the reading radiologist’s subjective interpretation. As such, these findings are often perplexing with high inter-observer variability, particularly in the third trimester, when T2 inhomogeneities are often found in the normal brain. Our current study’s objective was to overcome these factors by offering a quantitative, reproducible technique for assessing temporal lobes T2 signal on fbMRI in prenatal CMV infection.
Methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. IRB approval was granted by the ethical committee of Sheba Medical Center. A waiver for informed consent was approved by the ethical committee of Sheba Medical Center.
Study design and population (Figure 1)
Initially, a cohort of 68 normal fetuses, with no clinical or laboratory evidence of chromosomal abnormalities or intrauterine infection, that underwent fbMRI were reviewed for evaluation of the changes in the relative temporal lobe T2 signal intensity over the different gestational ages (study dates between 1/2017–6/2018). The indications for the fbMRI scan were the following: a sibling with perinatal neurologic findings, previous abnormal pregnancy, or prenatal sonography with abnormal findings not confirmed by fbMRI.
In Israel, the common practice is serial screening for CMV infection before or during the first trimester of pregnancy. Women who are seronegative in the first trimester are usually reassessed during the second and early third trimesters. A cohort of 51 fetuses suspected of intrauterine CMV infection following maternal CMV seroconversion was evaluated in the current study. These fetuses had fbMRI, following maternal seroconversion, at 32–33 weeks of gestation or earlier if brain lesions were suspected on a follow-up ultrasound examination.
Fetal\neonatal CMV infection was confirmed or excluded by viral DNA amplification using polymerase chain reaction (PCR) from the amniotic fluid (n=26) or newborns’ saliva or urine (n=43). Information regarding the performance of amniocentesis was collected from the mothers’ medical records. Of the suspected CMV cohort, viral PCR proved prenatal infection in 29 fetuses and excluded fetal infection in 22 fetuses. The clinical characteristics of CMV-suspected and normal control cohorts are presented in Table 1.

Full table
After birth, hearing evaluation with transient evoked otoacoustic emission (TEOAE) was performed in all newborns. A brainstem evoked response audiometry (BERA) was performed in all CMV-positive fetuses.
fbMRI
Scans were obtained using a 3-T MR system (Ingenia 3.0T, Philips, Netherlands). A single-shot fast spin-echo (SSFSE) T2-weighted sequence in three orthogonal planes was employed twice, with a slice thickness of 3–4 mm, with no gap, and using a dStream Torso coil (32-channels). The FOV was determined by the size of the fetal head and ranged from 24 cm for the smaller fetuses up to 28 cm for the larger ones. Other parameters were: matrix: 224/155; TE: 70 ms; TR: approximately 2,500 ms.
The mothers had refrained from eating or drinking fluids containing sugar for 4 hours before the fbMRI examination.
Image analysis
For measuring T2 signal within the temporal lobes, circular regions of interest (ROIs) of a constant size (50 mm2) were drawn using the PACS software (Vue PACS, Carestream, Rochester, NY, USA) on axial T2 images (Figure 2). The ROIs were drawn on an axial T2 image at the levels of the temporal poles. Mean and maximal intensity values were measured. There were no significant differences between the mean or maximal T2 values of the right and left temporal lobes (P=0.86 and 0.68, respectively). For the calculation, the average combining the right and left temporal lobes T2 signal was used (i.e., mean temporal T2 signal). The maximal value of both sides was also used (i.e., maximal temporal T2 signal). For normalization, ratios of mean or maximal temporal T2 signal were measured relative to the mean T2 signal within the amniotic fluid, which was used as an internal reference. A circular ROI of a constant size (30 mm2) was drawn in the amniotic fluid near the fetal head with no flow or shading artifact (Figure 2). The reading radiologists (L.G. and S.S.) were blinded to clinical data.

Additional findings, including qualitative temporal lobes T2 hyperintensities, ventriculomegaly, asymmetry of the lateral ventricles, or temporal periventricular cysts were also recorded from the formal radiology report (these findings were read in a consensus of two senior neuroradiologists, blinded to the CMV PCR analysis (S.S., E.K. or C.H.).
Interobserver validity of T2 signal measurements
To validate the consistency of measurements and reliability of the results, two observers evaluated the first consecutive 20 fetuses (L.G. and S.S). Interobserver and intra-observer variability were assessed by the interclass correlation coefficient (ICC). We considered an ICC value of >0.8 as an excellent agreement.
Statistical analysis
Categoric variables were expressed as number and percentage. Continuous variables were expressed as mean and standard deviation. The distribution of continuous variables was assessed using a histogram and Q-Q plot. Categoric variables were compared using the Chi-square test. Continuous variables were compared using the Student t-test or Kruskal Wallis Test. Intraclass correlation was used to assess inter- and intra-observer variability. Levene’s Test for Equality of Variances, Pearson, and Spearman’s were performed to analyze the correlations. ROC analysis was performed to evaluate discriminative ability. A 2-tailed P<0.05 was considered statistically significant. Analyses were performed with SPSS (Version 25.0, 2019; IBM, Armonk, New York).
Results
Interobserver validity of T2 signal measurement
Mean and maximal temporal lobes T2 signal and amniotic fluid mean T2 signal measurements showed excellent intra-observer and inter-observer agreement with the ICC above 0.88 and above 0.81, respectively.
Temporal lobes T2 hyperintensities
Quantitative assessment
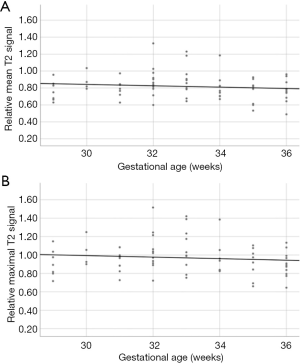
In the normal cohort, relative maximal and mean temporal lobe T2 signal have not changed significantly throughout the evaluated gestational age with P=0.345 and 0.245, respectively (Figure 3).
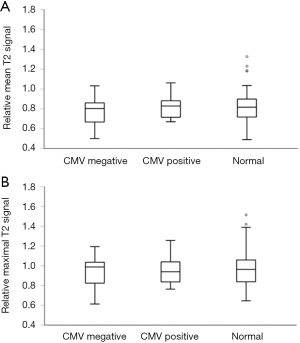
In our study cohort, there were no significant differences in the relative mean and maximal temporal lobes T2 signal intensities between the CMV positive, CMV negative, and normal control groups (P=0.68 and 0.92, respectively, Figure 4). Both mean and maximal relative temporal lobes T2 signal intensities did not have discriminative ability for fetal CMV status (area under the curve on ROC analysis was 0.57 and 0.51, respectively). An illustrative example of a significant overlap of T2 hyperintensity between CMV positive and negative fetuses is presented in Figure 5.

Qualitative assessment
Qualitative temporal lobe T2 hyperintensity (i.e., categorical assessment, as reported by the reading radiologists) was found in 9/29 fetuses with CMV infection and 2/22 fetuses without CMV infection (P=0.06, Table 2). This qualitative assessment was found to be in a positive correlation with the quantitative T2 signal assessment (r=0.44, P=0.001, for correlation with relative mean temporal lobes T2 signal, and r=0.45, P=0.01, for correlation with relative maximal temporal lobes T2 signal).

Full table
Additional MR imaging findings
In 5 fetuses with suspected CMV infection, temporal poles periventricular cysts were found. All of these fetuses were found to be infected with CMV. None of the fetuses in the CMV negative group have demonstrated this finding (P=0.04).
Asymmetric normal-sized lateral ventricles were noted in 5 fetuses; 3/29 CMV positive and 2/22 CMV negative fetuses had this finding. One fetus had moderate ventriculomegaly with sub-ependymal cysts (CMV positive). Two fetuses showed facial abnormalities (1 cleft lip in a CMV-positive and 1 with dacryocystocele in a CMV-negative fetus (Table 2). None of the CMV-positive fetuses showed any cortical gyration abnormalities.
Hearing impairment
Of the CMV-positive fetuses in our study cohort, two fetuses showed sensorineural hearing loss (SNHL). Due to the small number of fetuses with hearing impairment, statistical analysis of imaging-clinical correlation is limited. Temporal T2 signal corresponded to 28th and 35th percentiles (relative temporal mean and maximal T2 signal, respectively) in one fetus and 69th and 72nd in the other fetus (relative temporal mean and maximal T2 signal, respectively). One of these fetuses was reported on the qualitative analysis as showing an increased temporal pole T2 signal, whereas the second fetus was reported as normal with no parenchymal signal abnormality.
Discussion
In our study cohort of fetuses suspected to be infected with CMV, based on maternal seroconversion, no significant quantitative signal changes were found between PCR-proven CMV positive, CMV negative fetuses, or age-matched controls. Reporting abnormal increased T2 signal intensities in the polar temporal regions is not uncommon, especially when interpreting fbMRI of suspected fetal CMV infection with a low threshold for temporal lobe abnormalities (24,25). However, when evaluating this subjective assessment quantitatively, there was significant overlap with normal non-CMV infected fetuses. Consequently, isolated T2 hyperintensity, as an imaging finding suspected for CMV-related parenchymal injury, should be interpreted carefully.
The complexity of interpreting the temporal lobes T2 signal in CMV infected fetuses can be explained by overlapping between physiologic T2 signal hyperintensity and heterogenicity of the non-myelinated fetal WM and the temporal lobe CMV-related WM damage. Relative temporal lobes T2 signal intensities did not have discriminative power for fetal CMV status (ROC analysis). We found a good correlation between quantitative temporal poles T2 signal and expert qualitative assessment in the current study. Nevertheless, when analyzing qualitative imaging findings in conjunction with the PCR results of CMV infection, the sensitivity of the qualitative assessment of temporal lobes T2 hyperintensity was low (0.31). Recently, Birnbaum et al. showed a relatively high false positive rate for congenital CMV infection using fbMRI, with all false positive fetuses showing WM hyperintensities (26). This low sensitivity might also be related to the reading radiologist’s low threshold for temporal lobe abnormalities while reading fbMR study in maternal CMV infection cases and in fear of missing a potential CNS lesion (though reading radiologists were blinded to the actual fetal CMV status). The current study illustrates the importance of quantitative imaging in clinical practice.
The most common fbMRI findings in maternal CMV infection is WM T2 hyperintensity, suspicious for WMD (20,21). Previous studies have shown that the polar temporal regions’ abnormalities are characteristic of congenital CMV infection on postnatal MR imaging (16). These signal changes might represent edematous and inflammatory infiltration in the acute phase of CMV infection. At a later stage of infection, it might be secondary to residual gliosis and CMV-positive cells with inclusion bodies (27). Another hypothesis is that CMV infection of the placenta results in hypoxic changes and inflammatory infiltration within the cerebral WM, evident as WM T2 hyperintensity on fbMRI (27). Previous reports have demonstrated a significant correlation between brain MRI WM lesions and neurodevelopmental impairment in CMV infected fetuses that were symptomatic at birth (28,29). Cannie et al. have reported that CMV infected fetuses with temporal lobes T2 hyperintensities had up to 14% of SNHL, thus might represent CMV-related parenchymal injury (24). Nevertheless, Birnbaum et al. have reported that CMV infected fetuses with isolated temporal lobes WM T2 hyperintensities with otherwise normal fbMRI and normal serial neurosonography usually have a normal outcome (30). In the current study, we also did not find a correlation between neonatal SNHL and temporal lobes T2 signal (quantitatively and qualitatively). Specifically, when evaluating late CMV infection (second or third trimester), Elkan Miller et al. recently showed that prenatal fbMRI findings (near-exclusive WM signal abnormalities) were not significantly correlated with either hearing loss or neurodevelopmental abnormalities (31).
fbMRI is thought to be more sensitive in the early detection of structural pathology related to CMV infection in the fetal brain than ultrasound, which has implications in the prognostic evaluation and pregnancy management (32). In our cohort, the suspicion for CMV infection was based on maternal seroconversion with no significant fetal brain structural abnormalities (i.e., probably related to late maternal CMV infection). The most common associated abnormality was temporal poles cystic changes, which significantly increased the specificity for CMV infection (up to 100%). This was consistent with prior reports (13,32). Nevertheless, only a minority of the CMV infected fetuses had this finding (n=5), i.e., the sensitivity is relatively low. Other abnormalities, such as the asymmetry of the ventricles, did not have a significant diagnostic value.
In clinical practice, qualitative assessment of the signal intensity on T1 and T2 weighted sequences depends on the radiologist’s subjective interpretation, usually without any objective measurements. Assessment of quantitative tissue signal intensity on MRI is also potentially subject to variations, as it lacks inherent meaning and is influenced by sequence parameters and hardware selection. Thus, in the current study, the T2 signal within the temporal poles was quantitively measured and normalized to the amniotic fluid’s surrounding signal, enabling a relatively objective assessment of the T2 signal. Such a quantitative analysis of signal intensity, which is easily reproducible and comparable, can help standardize these findings for clinical use and future research. Similar relative T1 and T2 signal normalization and measurements have been reported in previous studies (33,34). Additional advanced MR sequences should be pursued to enable precise and quantitative assessment of parenchymal microstructure. Such methods would improve our detection of CMV-related injury and assist in pregnancy consulting and risk prognostication.
A significant limitation of our study, which stems from its retrospective design, is the absence of correlation with the neurodevelopmental outcome of fetuses with and without temporal poles signal abnormalities. Other limitations are the lack of pathological correlation for all our radiographic findings and the absence of information on viral load, affecting parenchymal injury (35).
Conclusions
Although described in the literature as an associated imaging finding in prenatal CMV infection, T2 signal in the temporal lobes does not differ significantly between CMV-positive and CMV-negative fetuses, probably overlapping with normal intrinsic relatively high T2 signal. Subjectively reported temporal T2 hyperintensities should be interpreted carefully and should have a limited effect on pregnancy management, especially when it is an isolated finding. Our study illustrates the importance of quantitative imaging in diagnostic neuroradiology.
Acknowledgments
The authors gratefully acknowledge Michael J. Wolf for his valuable assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-21-22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. IRB approval was granted by the ethical committee of Sheba Medical Center. A waiver for informed consent was approved by the ethical committee of Sheba Medical Center.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine Transmission of Cytomegalovirus to Infants of Women with Preconceptional Immunity. N Engl J Med 2001;344:1366-71. [Crossref] [PubMed]
- Kenneson A, Cannon MJ. Review and Meta-Analysis of the Epidemiology of Congenital Cytomegalovirus (CMV) Infection. Rev Med Virol 2007;17:253-76. [Crossref] [PubMed]
- Ivarsson SA, Lernmark B, Svanberg L. Ten-Year Clinical, Developmental and Intellectual Follow-up of Children with Congenital Cytomegalovirus Infection without Neurologic Symptoms at One Year of Age. Pediatrics 1997;99:800-3. [Crossref] [PubMed]
- Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 2009;22:99-126. [Crossref] [PubMed]
- Schleiss MR, Permar SR, Plotkin SA. Progress toward development of a vaccine against congenital cytomegalovirus infection. Clin Vaccine Immunol 2017;24:e00268-17. [Crossref] [PubMed]
- Gabrielli L, Bonasoni MP, Santini D, Piccirilli G, Chiereghin A, Petrisli E, Dolcetti R, Guerra B, Piccioli M, Lanari M, Landini MP, Lazzarotto T. Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin Microbiol Infect 2012;18:E419-E427. [Crossref] [PubMed]
- Shinmura Y, Kosugi I, Aiba-Masago S, Baba S, Yong LR, Tsutsui Y. Disordered migration and loss of virus-infected neuronal cells in developing mouse brains infected with murine cytomegalovirus. Acta Neuropathol 1997;93:551-7. [Crossref] [PubMed]
- Giannattasio A, Bruzzese D, Di Costanzo P, Capone E, Romano A, D’Amico A, Bravaccio C, Grande C, Capasso L, Raimondi F. Neuroimaging Profiles and Neurodevelopmental Outcome in Infants With Congenital Cytomegalovirus Infection. Pediatr Infect Dis J 2018;37:1028-33. [Crossref] [PubMed]
- Fink KR, Thapa MM, Ishak GE, Pruthi S. Neuroimaging of Pediatric Central Nervous System Cytomegalovirus Infection. Radiographics 2010;30:1779-96. [Crossref] [PubMed]
- Picone O, Costa JM, Ville Y, Chaix ML, Rouzioux C, Ville ML. Genetic Polymorphism of Cytomegalovirus Strains Responsible of Congenital Infections. Pathol Biol 2004;52:534-9. [Crossref] [PubMed]
- Malinger G, Lev D, Zahalka N, Ben Aroia Z, Watember N, Kidron D, Ben Sira L, Lerman-Sagie T. Fetal cytomegalovirus infection of the brain: the spectrum of sonographic findings. AJNR Am J Neuroradiol 2003;24:28-32. [PubMed]
- Leruez-Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol 2017;38:97-107. [Crossref] [PubMed]
- Averill LW, Kandula VV, Akyol Y, Epelman M. Fetal Brain Magnetic resonance imaging findings in congenital cytomegalovirus infection with postnatal imaging correlation. Semin Ultrasound CT MR 2015;36:476-86. [Crossref] [PubMed]
- Barkovich AJ, Lindan CE. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am J Neuroradiol 1994;15:703-15. [PubMed]
- Mutnal MB, Cheeran MC, Hu S, Lokensgard JR. Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One 2011;6:e16211 [Crossref] [PubMed]
- van der Knaap MS, Vermeulen G, Barkhof F, Hart AA, Loeber JG, Weel JF. Pattern of White Matter Abnormalities at MR Imaging: Use of Polymerase Chain Reaction Testing of Guthrie Cards to Link Pattern with Congenital Cytomegalovirus Infection. Radiology 2004;230:529-36. [Crossref] [PubMed]
- Steinlin MI, Nadal D, Eich GF, Martin E, Boltshauser EJ. Late intrauterine Cytomegalovirus infection: clinical and neuroimaging findings. Pediatr Neurol 1996;15:249-53. [Crossref] [PubMed]
- Guerra B, Simonazzi G, Puccetti C, Lanari M, Farina A, Lazzarotto T, Rizzo N. Ultrasound Prediction of Symptomatic Congenital Cytomegalovirus Infection. Am J Obstet Gynecol 2008;198:380.e1-7. [Crossref] [PubMed]
- Lucignani G, Rossi Espagnet MC, Napolitano A, Talamanca LF, Calo Carducci FI, Auriti C, Longo D. A New MRI Severity Score to Predict Long-Term Adverse Neurologic Outcomes in Children with Congenital Cytomegalovirus Infection. J Matern Fetal Neonatal Med 2021;34:859-66. [Crossref] [PubMed]
- Picone O, Simon I, Benachi A, Brunelle F, Sonigo P. Comparison between ultrasound and magnetic resonance imaging in assessment of fetal cytomegalovirus infection. Prenat Diagn 2008;28:753-8. [Crossref] [PubMed]
- Lipitz S, Hoffmann C, Feldman B, Tepperberg-Dikawa M, Schiff E, Weisz B. Value of prenatal ultrasound and magnetic resonance imaging in assessment of congenital primary cytomegalovirus infection. Ultrasound Obstet Gynecol 2010;36:709-17. [Crossref] [PubMed]
- Malinger G, Lev D, Lerman-Sagie T. Imaging of Fetal Cytomegalovirus Infection. Fetal Diagn Ther 2011;29:117-26. [Crossref] [PubMed]
- Buca D, Di Mascio D, Rizzo G, Giancotti A, D'Amico A, Leombroni M, Makatsarya A, Familiari A, Liberati M, Nappi L, Flacco ME, Manzoli L, Salomon LJ, Scambia G, D'Antonio F. Outcome of Fetuses with Congenital Cytomegalovirus Infection and Normal Ultrasound at Diagnosis: Systematic Review and Meta-Analysis. Ultrasound Obstet Gynecol 2021;57:551-9. [Crossref] [PubMed]
- Cannie MM, Devlieger R, Leyder M, Claus F, Leus A, De Catte L, Cossey V, Foulon I, Van der Valk E, Foulon W, Cos T, Bernaert A, Oyen R, Jani JC. Congenital Cytomegalovirus Infection: Contribution and Best Timing of Prenatal MR Imaging. Eur Radiol 2016;26:3760-9. [Crossref] [PubMed]
- Katorza E, Strauss G, Cohen R, Berkenstadt M, Hoffmann C, Achiron R, Barzilay E, Bar-Yosef O. Apparent Diffusion Coefficient Levels and Neurodevelopmental Outcome in Fetuses with Brain MR Imaging White Matter Hyperintense Signal. AJNR Am J Neuroradiol 2018;39:1926-31. [Crossref] [PubMed]
- Roee B, Adi W, Michael B, Igal W, Karina KH, Liat BS, Gustavo M. Subtle findings on fetal brain imaging in CMV infected pregnancies: What is the clinical significance? A retrospective analysis with outcome correlation. Prenat Diagn 2020;40:447-53. [Crossref] [PubMed]
- Capretti MG, Lanari M, Tani G, Ancora G, Sciutti R, Marsico C, Lazzarotto T, Gabrielli L, Guerra B, Corvaglia L, Faldella G. Role of Cerebral Ultrasound and Magnetic Resonance Imaging in Newborns with Congenital Cytomegalovirus Infection. Brain Dev 2014;36:203-11. [Crossref] [PubMed]
- Alarcon A, Martinez-Biarge M, Cabanas F, Quero J, Garcia-Alix A. A Prognostic Neonatal Neuroimaging Scale for Symptomatic Congenital Cytomegalovirus Infection. Neonatology 2016;110:277-85. [Crossref] [PubMed]
- Inaba Y, Motobayashi M, Nishioka M, Kaneko T, Yamauchi S, Kawasaki Y, Shiba N, Nishio S, Moteki H, Miyagawa M, Takumi Y, Usami S, Koike K. Correlation between White Matter Lesions and Intelligence Quotient in Patients with Congenital Cytomegalovirus Infection. Pediatr Neurol 2016;55:52-7. [Crossref] [PubMed]
- Birnbaum R, Ben-Sira L, Lerman-Sagie T, Malinger G. The Use of Fetal Neurosonography and Brain MRI in Cases of Cytomegalovirus Infection during Pregnancy: A Retrospective Analysis with Outcome Correlation. Prenat Diagn 2017;37:1335-42. [Crossref] [PubMed]
- Elkan Miller T, Weisz B, Yinon Y, Weissbach T, De Castro H, Avnet H, Hoffman C, Katorza E, Lipitz S. Congenital Cytomegalovirus Infection Following Second and Third Trimester Maternal Infection Is Associated With Mild Childhood Adverse Outcome Not Predicted by Prenatal Imaging. J Pediatric Infect Dis Soc 2021; [Crossref] [PubMed]
- Doneda C, Parazzini C, Righini A, Rustico M, Tassis B, Fabbri E, Arrigoni F, Consonni D, Triulzi F. Early Cerebral Lesions in Cytomegalovirus Infection: Prenatal MR Imaging. Radiology 2010;255:613-21. [Crossref] [PubMed]
- Coulthard A, Hall K, English P T, Ince PG, Burn DJ, Bates D. Quantitative analysis of MRI signal intensity in new variant Creutzfeldt-Jakob disease. Br J Radiol 1999;72:742-8. [Crossref] [PubMed]
- McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial Gadolinium Deposition after Contrast-Enhanced MR Imaging. Radiology 2015;275:772-82. [Crossref] [PubMed]
- Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini MP. Prenatal Indicators of Congenital Cytomegalovirus Infection. J Pediatr 2000;137:90-5. [Crossref] [PubMed]



