
Intrapatient repeatability of background 18F-FDG uptake on PET/CT
Introduction
Positron emission tomography with 2-deoxy-2-[fluorine-18] fluoro-D-glucose integrated with computed tomography (18F-FDG PET/CT) is increasingly important in the evaluation of the efficacy of treatment in lymphoma and many solid tumors. For an accurate interpretation of treatment response, the choice of an appropriate background is of vital importance. In clinical practice, the standardized uptake value (SUV) of background tissues such as the aortic blood pool (ABP) and liver are often used as references (1-3). However, variations in biological factors such as the blood glucose level (BGL), tracer uptake period, etc., may affect the 18F-FDG uptake of these regions (4). This can be problematic when defining a metabolic response, especially for quantitative evaluation, by the PET Response Criteria in Solid Tumors (PERCIST). Indeed, PERCIST requires that a measurable tumor uptake must be determined relative to the activity of a background. Understanding intrapatient repeatability of different background SUVs is important for serial comparison (either quantitative or visual) within the same patient.
Previous studies have investigated this issue of background SUV repeatability within a variable time interval. They appear to contain factors that could bias the results (5,6). These include the failure to consider tumor burden, therapeutic regimes, or other physiological factors (i.e., changes in body habitus), which may impact the background SUV (7-9). Our objective was to find the preferred background by assessing the repeatability of its activity on the same patient and scanner within several days. It was assumed that the patient habitus and tumor burden would remain stable during this time interval. The background activity was expressed by the standardized uptake value normalized to lean body mass (SUL). We also investigated the effect of various factors on the SULs.
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our Institutional Review Board (Sichuan University West China Hospital Review Board) approved the study. Our study was retrospective, so the requirement to obtain written informed consent concerning the study was waived, but all patients signed informed consent before undergoing PET/CT scans.
Patient selection
Patients who had repeat 18F-FDG PET/CT scans within 1 to 4 days were included. The repeat scans were required when the original scan was uninterpretable due to proven movement artifacts in the head and neck or gastrointestinal tract. These artifacts, however, did not affect the background SUL measurements. The inclusion criteria were as follows: adults, no background SUL measurement impact from an artifact, utilization of the same PET/CT scanner, normal liver and renal function, and normal white blood cell count. Patients were excluded from the study if they had diabetes, tumor involvement in regions of interest (ROI) in the background, liver radiotherapy, or 18F-FDG extravasation at the time of administration, and those who received any treatment in the interval between the two scans.
PET/CT protocol
Patients fasted for at least 6 hours before receiving an intravenous administration of 18F-FDG (5.5 MBq/kg of body weight). 18F-FDG was produced by an on-site cyclotron (Sumitomo Heavy Industries, Ltd., Japan) and an automated synthesizer (Allinone, Trasis, Belgium) and qualified by thin-layer chromatography with a radio-chemical purity greater than 98.0%. Patients were required to consume 1 L of water after the tracer injection. The attenuation CT scan was performed using a low-dose (120 kV, 40 kV, 40 mAs, slice thickness of 5 mm, and a pixel size of 1.2 mm × 1.2 mm) non-contrast protocol for the attenuation and localization of abnormal 18F-FDG activity. The PET images were acquired from mid-thigh to vertex at a speed of 1.5 min per bed and reconstructed using a line-of-response row-action maximum likelihood algorithm (3 iterations and 33 subsets, a slice thickness of 4 mm, pixel size of 4 mm × 4 mm, with no additional Gaussian smoothing). All of the scans were performed using a Gemini GXL PET/CT scanner (Philips, Netherlands).
Background SUL measurements
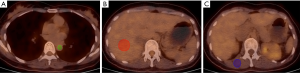
Background mean SUVs from the ABP, liver, and muscle were recorded. ABP activity was calculated by drawing three circular regions of interest (ROI) on three contiguous slices within the lumen of the thoracic aorta, taking care not to include the vessel wall in the ROI (Figure 1A). The liver 18F-FDG SUV was determined manually by placing an ROI with a diameter of 3 cm by 2 cm volume in the right hepatic lobe at the level of the main portal vein (Figure 1B). The muscle SUV was determined by selecting an ROI in the erector spine at the level of the 12th thoracic vertebra, including three contiguous slices within the margin of the muscle (Figure 1C). All operations were conducted by the post-processing software of the PET/CT viewer (Philips, Netherlands). SUVs were then normalized for lean body mass (LBM) using the following formulas (10,11):
- SUL = ActVOI (kBq/mL)/Actadministered (MBq)/LBM (kg);
- LBMmale = 9,270 × weight/(6,680 + 216 × BMI);
- LBMfemale = 9,270 × weight/(8,780 + 244 × BMI);
- All measurement data were expressed as mean ± standard deviation.
Statistical analysis
The patient variable factors, which included a finger stick BGL (mmol/L), injected tracer dose (MBq), tracer uptake period (min) between injection and ROI acquisition on PET, and mean SULs from the ABP (ABP-SUL), liver (L-SUL), and muscle (M-SUL) between the two scans were compared using paired Student t-tests. The repeatability of all SULs between the two scans was calculated using intraclass correlation coefficients (ICCs) generated by a two-way random-effects model with an absolute agreement definition and the coefficient of variation (CV), then displayed graphically by Bland-Altman plots. The ICCs of the ABP-SUL, L-SUL, and M-SUL were compared by pairwise comparison using MedCalc Statistical Software (version 15.2.2, MedCalc Software, Ostend, Belgium). Then, the intrapatient variations in SULs and factors were calculated as absolute variations and relative variations. An absolute variation was equal to the SUL in the second scan minus the SUL in the first scan. A relative variation was defined as the percentage of absolute variation as a proportion of the SUL of the first scan. We thought there might be an 18F-FDG equilibration between tissue and blood (12,13), so the different background SUL variations were assessed using a Pearson correlation to confirm if they had the same direction. Finally, a linear regression model was used to analyze all of the relative variations to identify which factors correlated with the SUL variability. SPSS 19.0 software (SPSS, Chicago, IL, USA) was used for the statistical analyses. A P value <0.05 was considered statistically significant.
Results
Patient characteristics, SULs, and factors of each scan
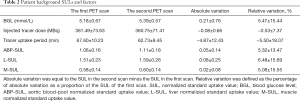
Thirty patients [19 males and 11 females, 20–80 (49.80±15.42) years] were included based on the inclusion and exclusion criteria. They included 11 with lymphoma, 7 undergoing cancer screening, and 12 with other malignancies. The mean time interval was 1–4 (2.23±1.04) days for the two scans. There were no significant variations between the two scans concerning the BGL, injected dose, and tracer uptake period (all P>0.05). Similarly, there were no significant variations between the two scans concerning any of the background SULs (all P>0.05). Tables 1 and 2 give an overview of patient characteristics, SULs, and factors.

Full table
Full table
Intrapatient repeatability for SULs
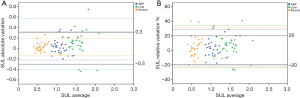
The ICCs for the ABP, liver, and muscle were 0.65 (95% CI, 0.38–0.81), 0.47 (95% CI, 0.15–0.70), and 0.82 (95% CI, 0.65–0.91), respectively. The M-SUL ICC was higher than that of the liver (P=0.02). The ABP-SUL ICC tended to be higher than that of the liver but was not significantly different (P=0.33). The ABP-SUL, L-SUL, and M-SUL CVs were 9%, 12%, and 10%, respectively. In the Bland-Altman plots (Figure 2), 23% (7/30) of the absolute variations and 23% (7/30) of the relative variations in the L-SUL were found outside the permitted variation limits by PERCIST. These numbers were 10% (3/30) and 10% (3/30) for the ABP-SUL, and 0% (0/30) and 7% (2/30) for the M-SUL, respectively.
Correlations between the variations of different background SULs
In our patient cohort, all of the background SUL variations between the two scans were in line with a Gaussian distribution. There was a moderate to strong positive correlation between the ABP-SUL and L-SUL with a Pearson coefficient of 0.48 (P<0.05). The same results were found between the L-SUL and M-SUL, and between the ABP-SUL and M-SUL, with Pearson coefficients of 0.48 (P<0.05) and 0.55 (P<0.05), respectively.
Correlations between SULs and each factor
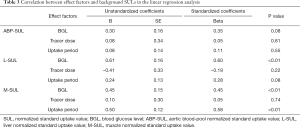
A linear regression analysis indicated there was a positive correlation between the variations of the L-SUL and the BGL (b=0.60, P<0.01). A similar result was found between the variations of the M-SUL and the BGL (b=0.45, P<0.01). The variation of the M-SUL also had a positive correlation with the variation of the tracer uptake period (b=0.58, P<0.01). There was no correlation between the variations of the ABP-SUL, the BGL, and tracer uptake period or between the variations of any background SULs and the tracer dose (all P>0.05). The effect of the BGL was more significant on the L-SUL than on the M-SUL (b=0.60 and 0.45, respectively). For the M-SUL, the effect of the BGL was less significant than that of the 18F-FDG uptake period (b=0.45 and 0.58, respectively; Table 3).
Full table
The repeatability of different background SUVs and their correlation with each factor
We also analyzed the repeatability of different background SUVs and their correlation with each factor and found similar results. These detailed results are shown in Tables S1,S2 and Figure S1.
Discussion
Understanding the repeatability of different background SULs and their impact is vital for an accurate response assessment on 18F-FDG PET/CT. Instead of a relative magnitude in previous studies, the absolute SUVs on serial scans were used to calculate variation (14,15). However, one SUV unit change in a patient with a low background SUV does not have the same significance as that of a high background SUV.
From our study, we found that the intrapatient liver SUL had a weak agreement. This was consistent with the results of a test-retest study by Tahari et al., who evaluated the repeatability of the liver SUL in the same patients at two-time points (the time interval was 235±192 days). The authors found that there was only fair repeatability of the liver SUL in the same patient in a clinical setting. The ICCs were 0.37 and 0.38 at the portal level for the two readers (16). Some other previous studies have also reported similar results because the mediastinum 18F-FDG uptake was more stable than the liver 18F-FDG uptake. These studies enrolled patients undergoing multiple 18F-FDG PET/CT exams during chemotherapy (5,17). However, the lengthy-time interval between the two 18F-FDG PET/CT exams (where the patient's underlying condition may be different), the variation in tumor burden, and ongoing systemic chemotherapy may have affected the 18F-FDG uptake in normal tissues. In contrast, our study had a short time interval (the mean time interval was 2 days), no additional chemotherapy was administered between the scans, and there was a more stable tumor burden. So, our results are likely to reflect the true characteristics of the repeatability of different background SULs.
We found the ABP-SUL and L-SUL variations were positively correlated, as were the variations between the L-SUL and M-SUL and the ABP-SUL and M-SUL. If significant and opposite correlations are noticed, the background choice should be treated with caution because one or both of the variations might reflect disease or abnormality. This phenomenon might be found in patients with hyperthyroidism, where the patient will have a decreased L-SUL but an increased M-SUL (18).
Our study indicated that there are significant positive associations between BGLs and the L-SUL and between BGLs and the M-SUL. This finding was in line with previous studies (19-23). The mechanism is not clear. Possible explanations are that a patient with a higher BGL may trap more 18F-FDG in the liver, which results in an increased SUL. A higher BGL may also evoke increased endogenous insulin with an enhanced 18F-FDG uptake in the liver and muscular tissue (19,24-28). Also, we found that the effect of BGLs on the L-SUL was greater than that on the M-SUL. This may be attributed to the liver’s crucial role in maintaining the homeostasis of the BGL under various physiological states (29,30). Some previous investigations of these correlations have shown different results. The reason may be that their data included diabetes patients who showed dysregulation in the liver and muscle uptake of blood glucose (21,26,31).
As a glucose analog, 18F-FDG is transported into the cells and gets trapped there after phosphorylation. In the presence of glucose-6-phosphatase, the 18F-FDG-6-phosphate can be dephosphorylated back to 18F-FDG and released into the bloodstream. Because this enzyme is minimally present in skeletal muscle fibers, a higher M-SUL after a longer uptake period is also concordant with expected results (32). Although the liver is rich in glucose-6-phosphatase, a kinetic model analysis revealed that the liver SUL could be considered nearly constant between 50 and 110 min after tracer injection (33). Therefore, the impact of the limited variation in the tracer uptake period between the two scans can be disregarded.
As opposed to the L-SUL and M-SUL, the ABP-SUL has stability in the face of any change in the BGL and period of tracer uptake. Because of the 18F-FDG equilibration between the liver and blood, the ABP-SUL will correlate positively with the L-SUL, as demonstrated by our study. However, it has been shown that the liver to blood ratio will increase due to a glucose-induced increase in 18F-FDG phosphorylation after a glucose load. This may explain why the BGL has a significant positive impact on the L-SUL rather than on the ABP-SUL (34). As with the liver, a time window may exist in which a temporary dynamic equilibrium can be reached between the rate of 18F-FDG plasma clearance and glucose-6-phosphatase-rich organ release. Within this time window, the ABP-SUL could be considered nearly constant. However, we felt that there must exist an 18F-FDG uptake variability on different scans in normal tissues in any single subject, even under technically and biologically ideal circumstances. Since SUL is normalized to an injected dose, no impact on background SUL from this variable is expected.
Limitations
The main limitation of our study is the relatively small sample size, which may have increased the statistical bias. Furthermore, the background activity may have been influenced by certain malignancies in selected patients, particularly lymphoma. However, because the interval between the two scans was so short (1–4 days), this influence should have been minimal. Though the required “limits” of background SULs in PERCIST imply equal uptake periods, no prospective attempt was made to assure similar uptake times in this study. In a busy PET center with a high examination flow, it is not easy to ensure that individuals with complex conditions receive their scans on a schedule. So, our results need to be interpreted within the context of the study design. Lastly, the equations for estimating LBM were developed using Caucasian subjects. They may not be appropriate to apply to Chinese patients.
Conclusions
Activities within the ABP and muscle are more stable between two scans than those in the liver and should be the preferred background for sequential patient evaluation. The SUL of the liver is more sensitive to the BGL, which is difficult to keep consistent across different scans, and therefore the liver may not be suitable as a referential background.
Acknowledgments
Funding: This work was supported by grants from the Sichuan Provincial Department of Science and Technology support program (2015SZ0128), the National Key Research and Development Program of China during the “13th five-year plan” (2016YFC0104300), and the National Natural Science Foundation of China (81971653).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-769). The authors report that this work was supported by a grant from the Sichuan Provincial Department of Science and Technology support program (2015SZ0128), the National Key Research and Development Program of China during the “13th five-year plan” (2016YFC0104300), and the National Natural Science Foundation of China (81971653).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by our Institutional Review Board (Sichuan University West China Hospital Review Board). The study was retrospective, so the requirement to obtain written informed consent concerning the study was waived, but all patients signed informed consent before undergoing the PET/CT scans.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50:122S-50S. [Crossref] [PubMed]
- Marcus C, Ciarallo A, Tahari AK, Mena E, Koch W, Wahl RL, Kiess AP, Kang H, Subramaniam RM. Head and neck PET/CT: therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J Nucl Med 2014;55:1411-6. [Crossref] [PubMed]
- Barrington SF MN, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol 2014;32:3048-58. [Crossref] [PubMed]
- Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 2010;195:310-20. [Crossref] [PubMed]
- Chiaravalloti A, Danieli R, Abbatiello P, Di Pietro B, Travascio L, Cantonetti M, Guazzaroni M, Orlacchio A, Simonetti G, Schillaci O. Factors affecting intrapatient liver and mediastinal blood pool 18F-FDG standardized uptake value changes during ABVD chemotherapy in Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging 2014;41:1123-32. [Crossref] [PubMed]
- Kim SJ, Yi HK, Lim CH, Cho YS, Choi JY, Choe YS, Lee KH, Kim BT, Moon SH. Intra-patient variability of FDG standardized uptake values in mediastinal blood pool, liver, and myocardium during R-CHOP chemotherapy in patients with diffuse large B-cell lymphoma. Nucl Med Mol Imaging 2016;50:300-7. [Crossref] [PubMed]
- Mahmud MH, Mahmud MH NA, Ahmad Saad FF, Azman AZ. Impacts of biological and procedural factors on semiquantification uptake value of liver in fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography imaging. Quant Imaging Med Surg 2015;5:700-7. [PubMed]
- Sprinz C, Altmayer S, Zanon M, Watte G, Irion K, Marchiori E, Hochhegger B. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: a systematic review. PLoS One 2018;13:e0193140 [Crossref] [PubMed]
- Wang R, Chen H, Fan C. Impacts of time interval on 18F-FDG uptake for PET/CT in normal organs: a systematic review. Medicine (Baltimore) 2018;97:e13122 [Crossref] [PubMed]
- Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet 2005;44:1051-65. [Crossref] [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJEuropean Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]
- Chen DL, Mintun MA, Schuster DP. Comparison of methods to quantitate 18F-FDG uptake with PET during experimental acute lung injury. J Nucl Med 2004;45:1583-90. [PubMed]
- Keramida G, Anagnostopoulos CD, Peters AM. The extent to which standardized uptake values reflect FDG phosphorylation in the liver and spleen as functions of time after injection of (18)F-fluorodeoxyglucose. EJNMMI Res 2017;7:13. [Crossref] [PubMed]
- Boktor RR, Walker G, Stacey R, Gledhill S, Pitman AG. Reference range for intrapatient variability in blood-pool and liver SUV for 18F-FDG PET. J Nucl Med 2013;54:677-82. [Crossref] [PubMed]
- Groheux D, Delord M, Rubello D, Colletti PM, Nguyen ML, Hindie E. Variation of liver SUV on 18F-FDG-PET/CT studies in women with breast cancer. Clin Nucl Med 2013;38:422-5. [Crossref] [PubMed]
- Tahari AK, Paidpally V, Chirindel A, Wahl RL, Subramaniam RM. Two-time-point FDG PET/CT: liver SULmean repeatability. AJR Am J Roentgenol 2015;204:402-7. [Crossref] [PubMed]
- Ceriani L, Suriano S, Ruberto T, Zucca E, Giovanella L. 18F-FDG uptake changes in liver and mediastinum during chemotherapy in patients with diffuse large B-cell lymphoma. Clin Nucl Med 2012;37:949-52. [Crossref] [PubMed]
- Chen YK, Chen YL, Tsui CC, Wang SC, Cheng RH. The significance of alteration 2-[fluorine-18]fluoro-2-deoxy-(D)-glucose uptake in the liver and skeletal muscles of patients with hyperthyroidism. Acad Radiol 2013;20:1218-23. [Crossref] [PubMed]
- Iozzo P, Geisler F, Oikonen V, Maki M, Takala T, Solin O, Ferrannini E, Knuuti J, Nuutila P, Study FFP. Insulin stimulates liver glucose uptake in humans: an 18F-FDG PET study. J Nucl Med 2003;44:682-9. [PubMed]
- Kreissl MC, Stout DB, Wong KP, Wu HM, Caglayan E, Ladno W, Zhang X, Prior JO, Reiners C, Huang SC, Schelbert HR. Influence of dietary state and insulin on myocardial, skeletal muscle and brain [F]-fluorodeoxyglucose kinetics in mice. EJNMMI Res 2011;1:8. [Crossref] [PubMed]
- Lindholm H, Brolin F, Jonsson C, Jacobsson H. The relation between the blood glucose level and the FDG uptake of tissues at normal PET examinations. EJNMMI Res 2013;3:50. [Crossref] [PubMed]
- Malladi A, Viner M, Jackson T, Mercier G, Subramaniam RM. PET/CT mediastinal and liver FDG uptake: effects of biological and procedural factors. J Med Imaging Radiat Oncol 2013;57:169-75. [Crossref] [PubMed]
- Tai SJ, Liu RS, Kuo YC, Hsu CY, Chen CH. Glucose uptake patterns in exercised skeletal muscles of elite male long-distance and short-distance runners. Chin J Physiol 2010;53:91-8. [Crossref] [PubMed]
- Büsing KA, Schönberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol 2013;40:206-13. [Crossref] [PubMed]
- Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC 3rd. Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation 1994;89:793-8. [Crossref] [PubMed]
- Wasserman DH KL, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol 2011;214:254-62. [Crossref] [PubMed]
- Webb RL, Landau E, Klein D, DiPoce J, Volkin D, Belman J, Voutsinas N, Brenner A. Effects of varying serum glucose levels on 18F-FDG biodistribution. Nucl Med Commun 2015;36:717-21. [Crossref] [PubMed]
- Kubota K, Watanabe H, Murata Y, Yukihiro M, Ito K, Morooka M, Minamimoto R, Hori A, Shibuya H. Effects of blood glucose level on FDG uptake by liver: a FDG-PET/CT study. Nucl Med Biol 2011;38:347-51. [Crossref] [PubMed]
- König M, Bulik S, Holzhütter HG. Quantifying the contribution of the liver to glucose homeostasis: a detailed kinetic model of human hepatic glucose metabolism. PLoS Comput Biol 2012;8:e1002577 [Crossref] [PubMed]
- Sherwin RS. Role of the liver in glucose homeostasis. Diabetes Care 1980;3:261-5. [Crossref] [PubMed]
- Hara T, Higashi T, Nakamoto Y, Suga T, Saga T, Ishimori T, Ishizu K, Kawashima H, Kawase S, Matsumoto K, Togashi K. Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging? Ann Nucl Med 2009;23:657-69. [Crossref] [PubMed]
- Hutton JC. OBR. Glucose-6-phosphatase catalytic subunit gene family. J Biol Chem 2009;284:29241-5. [Crossref] [PubMed]
- Laffon E, Adhoute X, de Clermont H, Marthan R. Is liver SUV stable over time in 18F-FDG PET imaging? J Nucl Med Technol 2011;39:258-63. [Crossref] [PubMed]
- Choi Y, Hawkins RA, Huang SC, Brunken RC, Hoh CK, Messa C, Nitzsche EU, Phelps ME, Schelbert HR. Evaluation of the effect of glucose ingestion and kinetic model configurations of FDG in the normal liver. J Nucl Med 1994;35:818-23. [PubMed]


