
Treatment of a special-located occult hepatic cancer in a cirrhotic patient using laparoscopic ultrasound-guided radiofrequency ablation: a case description
Introduction
Liver cancers are the sixth most common type of cancer worldwide and the fourth most frequent cause of cancer death (1). Radiofrequency ablation (RFA) is conventionally the first choice of treatment for hepatocellular carcinoma (HCC). When the tumor is smaller than 3 cm, the curative effect is similar to that of surgical resection (2). Herein, we have reported a case of a special-located HCC adjacent to the stomach, which was successfully treated using RFA under laparoscopic ultrasound without any serious complications.
Case presentation
All procedures performed in studies involving human participants were conducted following the institution’s ethical standards and with the Helsinki Declaration (as revised in 2013). The patient provided written informed consent.
A 56-year-old woman with hepatitis B virus-related cirrhosis and incomplete portal vein thrombosis was admitted to our hospital to treat HCC. The patient had undergone splenectomy 17 years prior for peripheral blood cell reduction and had felt fatigued for 4 months. After admission, contrast-enhanced computed tomography (CT) revealed an enhanced nodule measuring 12×10 mm in the left hepatic lobe in the arterial phase. Enhanced magnetic resonance imaging (MRI) showed that the nodule had arterial hyperenhancement and portal washout. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI confirmed the mass had arterial hyperintensity and hepatobiliary hypointensity, indicating HCC. Gray-scale ultrasound failed to display the nodule, while contrast-enhanced ultrasound (CEUS) under virtual-navigation revealed the nodule in the arterial phase. Alpha-fetoprotein, albumin, bilirubin, and prothrombin time were all normal. More than two imaging methods showed the patient’s nodule in the left hepatic lobe with arterial enhancement, suggesting HCC (3) (Figure 1A,B).
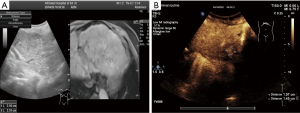
The performance of the tumor in ultrasound and CEUS is displayed in Figure 1A,B. In the background of liver cirrhosis, no obvious nodules were found on gray-scale ultrasound or a real-time ultrasound display screen with ultrasound and enhanced MRI image fusion virtual navigation (Figure 1A). After injection with sulfur hexafluoride for CEUS in peripheral intravenous, an enhanced nodule 16×15 mm in size was detected (Figure 1B).
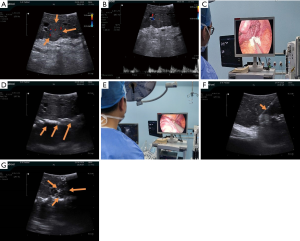
According to the Guidelines for diagnosis and treatment of primary liver cancer in China (3), we performed RFA of liver cancer under laparoscopic ultrasound. The tumor was revealed under the view of laparoscopic ultrasound (Figure 2A,B). Given that the tumor was small and near the stomach, we placed some wet gauze strips between it and the stomach to prevent thermal damage to the stomach wall during ablation (Figure 2C,D). A 250 W, 470 kHz bipolar radiofrequency generator (CelonLab POWER, Celon AG Medical Instruments, Teltow, Germany) was used as the energy source. A 16-gauge internally cooled, 20 mm active tip electrode was passed through a laparoscopic ultrasound puncture hole under the direct vision of laparoscope, then was inserted into the tumor in the plane under the guidance of laparoscopic ultrasound (Figure 2E,F). Real-time laparoscopic ultrasound monitored the whole ablation procedure (Figure 2G). The tumor was ablated until the internal gasification had been covered with a safe boundary of 0.5–1.0 cm from its margin. The whole ablation lasted 12 minutes. Finally, the electrode path was cauterized during electrode retraction. The operation was concluded end after confirming that there was no active bleeding or adjacent organ damage.
The patient was reexamined an enhanced MRI at 1 and 6 months after the operation to evaluate the effect of RFA. No enhancement was found in any of the 3 phases, suggesting tumor necrosis and no local progression (Figure 3).
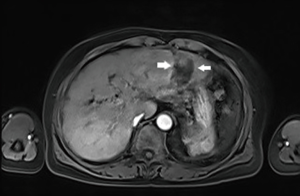
The imaging finding in enhanced MRI post-operation. Figure 3: at 6 months after the operation, no abnormal perfusion at the former site is visible.
Discussion
Liver tumor location is a key feature in determining the treatment of HCC. The lesion, in this case, was not detected with conventional ultrasound examination; only with the help of ultrasound-MRI image fusion virtual navigation, the nodule was located in the corresponding area by CEUS. In this case, percutaneous ablation was abandoned on account of the lesion’s proximity to the stomach. During the operation, laparoscopic ultrasonography clearly showed that the mass protruded from the visceral surface of the left liver lobe to the lesser omental sac of the stomach. Furthermore, laparoscopic color Doppler ultrasound detected that the blood flow velocity of the tumor’s feeding artery was 10 cm/s. Laparoscopic ultrasound can enable visualization of occult liver cancer. The stomach is the most frequently injured organ during percutaneous RFA for tumors of the left lateral hepatic segment. In our case, we inserted some wet gauze grips behind the tumor to protect the stomach wall, thus alleviating any concern regarding gastric perforation due to thermal injury during the ablation. In the present case, we performed RFA under laparoscopic ultrasound with satisfactory clinical outcomes.
Acknowledgments
Funding: This work was supported by the Department of Education of Zhejiang Province [Y202043215].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-21-207). All authors report that this work was supported by the Department of Education of Zhejiang Province [Y202043215].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were following the ethical standards of the institution and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- Sotiropoulos GC, Lang H, Frilling A, Molmenti EP, Paul A, Nadalin S, Radtke A, Brokalaki EI, Saner F, Hilgard P, Gerken G, Broelsch CE, Malagò M. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology 2006;53:322-9. [PubMed]
- Department of Medical Administration, National Health and Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi 2020;28:112-28. [PubMed]


