
Diagnostic accuracy of 18F-FDG PET/CT scan for peritoneal metastases in advanced ovarian cancer
Introduction
Ovarian cancer is one of the most common and lethal diseases among women around the world. At initial diagnosis, over two-thirds of patients are of advanced stage, with widespread intraabdominal disease (1,2). The standard treatment for ovarian cancer includes staging or debulking surgery and platinum-based adjuvant chemotherapy. Intraabdominal extent of ovarian cancer could influence treatment strategy and surgical outcomes.
Eisenkop score and peritoneal cancer index (PCI) are intraoperative ranking systems to quantify the tumor burden (3,4). These scores have been reported correlated with complete cytoreduction and patients’ survival (5,6). Thus, an accurate preoperative tumor burden assessment would be useful for appropriate treatment strategy selection and prognosis prediction.
18F-FDG PET/CT has been an important molecular imaging modality for preoperative and therapeutic effect assessment in ovarian cancer for several decades. Previous studies have shown that these semi-quantitative parameters are associated with patient clinical characteristics and prognosis in ovarian cancer (7-14). However, there is limited data showing diagnostic accuracy of PET/CT scans for peritoneal metastases in advanced ovarian cancer.
The aim of the present study was to assess the diagnostic accuracy of PET/CT to determine the Eisenkop score and PCI in correlation with surgical findings.
Methods
Data collection
This study was conducted according to the Declaration of Helsinki and was approved by the Committee at Fudan University Shanghai Cancer Center (FUSCC). All individual participants consented to the use of their medical records for research purposes. Between September 1, 2015, and February 28, 2018, forty-three patients underwent preoperative PET/CT scans, followed by primary cytoreductive surgery for advanced ovarian cancer. The histological diagnoses were based on WHO criteria. No patients included in this group received any disease treatment prior to referral, and the clinical data were prospectively collected.
18F-FDG PET/CT scan
All patients had PET/CT scans within 2 weeks before treatment. 18F-FDG was produced automatically by cyclotron (Siemens CTI RDS Eclipse ST, Knoxville, TN, USA) using the Explora FDG4 module in our center. Radiochemical purity was over 95%. All patients were asked to fast at least 6 hours before the 18F-FDG PET/CT procedure. Each patient’s blood glucose level was below 10 mmol/L at the time of radio-tracer injection. The standard dosage of intravenous 18F-FDG administration was 7.4 MBq/kg. Before and after injection, patients were kept lying comfortably in a quiet, dimly lit room. Examination was performed 60 minutes after 18F-FDG injection. A Siemens Biograph 16HR PET/CT scanner (Knoxville, TN, USA) with 4 mm transaxial intrinsic spatial resolution (full width at half maximum) and 16.2 cm axial field width was used for image scanning. Whole-body PET/CT data acquisition began with low-dose CT from the inguinal region to the head, with 120 kV, a 130–370 mA automatic adjustment, a pitch of 3.6, and a 0.5 second rotation time, followed by PET emission scan in a three-dimensional mode, with 2–3 minutes per bed position. The PET data were reconstructed using the ordered-subset expectation maximization technique selecting 8 subsets and 4 iterations, a 168×168 matrix. The CT data were used for attenuation correction of the PET images, and coregistered images were displayed on a workstation. The reconstructed images were then converted to a semiquantitative image corrected by the injection dose and the subject’s body weight [standardized uptake value (SUV)].
Tumor burden assessments and statistical analyses
Preoperative and intraoperative assessments of tumor burden [with Eisenkop (3) and PCI (4) score] were recorded by experienced radiologists and gynecological oncologists, respectively (Tables S1 and S2). Tumor volume was categorized according to Eisenkop score: 0–5 (small-volume tumor); 6–10 (moderate-volume tumor); and ≥11 (large-volume tumor).
SPSS statistical software (version 21.0, SPSS, IBM Inc., New York, USA) was used for the statistical analyses. Descriptive statistics were used for the demographic data and summarized as medians with ranges or frequencies with percentages. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for identifying peritoneal metastasis were calculated for each anatomical site.
Results
Patient characteristics
The patient characteristics are summarized in Table 1. A total of 43 advanced ovarian cancer patients were included in the analysis. The median [range] age was 57 [38–76] years old. The median CA125 was 774.8 U/mL. Thirty-two (74.4%) patients were diagnosed with stage III, and 11 (25.6%) patients were stage IV. Among these individuals, 19 (44.2%) patients had no residual disease after primary debulking surgery.
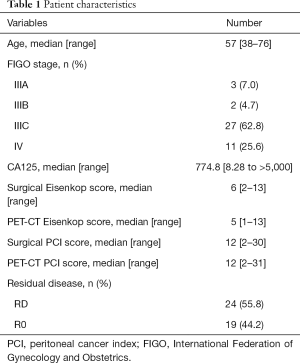
Full table
Performance of 18F-FDG PET/CT scan for preoperative assessment
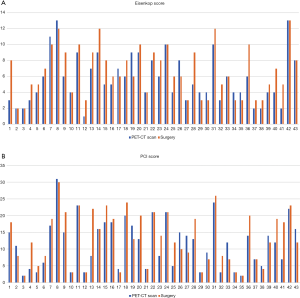
The median [range] Eisenkop score on PET/CT scan and surgical findings were 5 [1–13] and 6 [2–13], respectively. PET/CT scans correctly predicted the Eisenkop score with high sensitivity (84.2%), specificity (87.0%), and accuracy (85.1%) (Table 2 and Figure 1A). The median (range) PCI score on PET/CT scans and surgical findings were 12 [2–31] and 12 [2–30], respectively. The diagnostic accuracy of PET/CT scans for PCI scores was lower (78.5%), with 72.7% sensitivity and 84.9% specificity (Table 3 and Figure 1B).

Full table
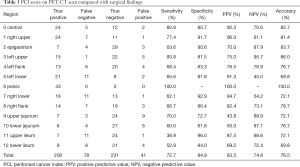
Full table
Compared to PCI, Eisenkop score could also evaluate retroperitoneal metastases. The diagnostic accuracy of retroperitoneum was up to 93%, with 89.5% sensitivity and 95.8% specificity. The diagnostic accuracy was low for jejunum and ileum by PCI assessment. Also, the predictive values for left flank, left lower flank, right flank, and right lower flank were unsatisfactory (Table 3). However, the area of central abdomen for Eisenkop score, which included both intestine and lateral pericolic gutters, had considerable diagnostic accuracy (81.4%), with 89.7% sensitivity and 64.3% specificity (Table 2). Besides, the sensitivity of the right upper quadrant was a bit lower in both ranking systems.
As for tumor volume, 34 patients were correctly categorized; 6 patients with moderate volume tumor were misclassified as small volume on PET/CT scan, and 2 patients with large volume tumor were assessed as moderate volume. While only 1 patient with moderate volume was recognized as large volume. Thus, preoperative PET/CT scans might underestimate tumor volume compared with surgical findings (Table 4).

Full table
Discussion
The present study validated two ranking systems for tumor burden on preoperative PET/CT scans and surgical findings in a prospective patient cohort. 18F-FDG PET/CT scans were not effective for preoperative PCI assessment in advanced ovarian cancer. That being said, it could accurately predict peritoneal metastases using Eisenkop score.
Advanced ovarian cancer is characterized by peritoneal dissemination, and the volume of residual disease is inversely correlated with the prognosis (15-17). Two ranking systems, Eisenkop score and PCI, have been reported to be correlated with patients’ surgical and survival outcomes (3-6). For patients with tumors too extensive to be adequately cytoreduced, appropriate preoperative imaging helps prevent unnecessary surgeries. Our study aimed to contribute to careful patient selection and preoperative treatment prediction.
CT used to be the most common preoperative method for ovarian cancer patients, with good specificity but poor sensitivity for identifying peritoneal metastases (18-21). Compared to conventional CT scan, 18F-FDG PET/CT can also provide functional or metabolic characteristics, and it has been used increasingly during ovarian cancer treatment (7-13). Hynninen et al. (22) demonstrated that PET/CT was superior to conventional CT for the detection of carcinomatosis in subdiaphragmatic peritoneal surfaces and bowel mesentery for advanced ovarian cancer. However, the sensitivity of both PET/CT and CT scans was poor in certain areas (64% vs. 27% in the small bowel mesentery and 65% vs. 55% in the right upper abdomen). Unlike previous studies, our study evaluated, for the first time, the diagnostic accuracy of PET/CT scans using two intraoperative ranking systems. Our data showed considerable sensitivity of PET/CT scans for peritoneal metastases in advanced ovarian cancer, with 84.2% for Eisenkop score and 72.7% for PCI.
We demonstrated that Eisenkop score performed better than PCI by preoperative PET/CT scan. The diagnostic accuracy of PET/CT by Eisenkop score was higher than that acquired using PCI score, with simple anatomical classification. For PCI assessment, it was hard to distinguish jejunum from ileum, especially to divide the intestines into four anatomical areas. Thus, the diagnostic accuracy was low for these parts. When considered as a whole, the diagnostic accuracy rose for the central abdomen by Eisenkop score. In addition, Eisenkop score could also correctly evaluate retroperitoneal metastases, although this was not included in the PCI assessment. However, the sensitivity of the right upper quadrant was a bit lower in both ranking systems because small-volume metastases on diaphragm and subcapsular liver metastases could be affected by physiological FDG distribution of liver. This may also lead to the result that tumor volume is underestimated by PET/CT scan.
The limitation of our study was the small number of enrolled patients. However, our systematic prospective data collection by the multidisciplinary team could compensate for that, making our results more reliable and applicable.
Our study suggested that 18F-FDG PET/CT scan accurately predicted peritoneal metastases in advanced ovarian cancer before surgery using Eisenkop score. PET/CT scans should be regarded as a helpful preoperative assessment method.
Acknowledgments
The authors would like to thank all doctors, nurses, patients, and their family members for their support of the present study.
Funding: This study was supported by the Guidance Project of Science and Technology Commission of Shanghai Municipality (No. 17411963000) for XH Wu.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-784). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Committee at Fudan University Shanghai Cancer Center (FUSCC). All individual participants consented to the use of their medical records for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2012;119:S118-29. [Crossref] [PubMed]
- Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol 2003;90:390-6. [Crossref] [PubMed]
- Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995;221:124-32. [Crossref] [PubMed]
- Fagotti A, Vizzielli G, Fanfani F, Scambia G. Comparison of peritoneal carcinomatosis scoring methods in predicting resectability and prognosis in advanced ovarian cancer. Am J Obstet Gynecol 2010;203:e10-1; author reply e1. [Crossref] [PubMed]
- Feng Z, Wen H, Jiang Z, Liu S, Ju X, Chen X, Xia L, Xu J, Bi R, Wu X. A triage strategy in advanced ovarian cancer management based on multiple predictive models for R0 resection: a prospective cohort study. J Gynecol Oncol 2018;29:e65 [Crossref] [PubMed]
- Risum S, Loft A, Hogdall C, Berthelsen AK, Hogdall E, Lundvall L, Nedergaard L, Engelholm SA. Standardized FDG uptake as a prognostic variable and as a predictor of incomplete cytoreduction in primary advanced ovarian cancer. Acta Oncol 2011;50:415-9. [Crossref] [PubMed]
- Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The pretreatment of maximum standardized uptake values (SUVmax) of the primary tumor is predictor for poor prognosis for patients with epithelial ovarian cancer. Acta Med Okayama 2012;66:53-60. [PubMed]
- Tanizaki Y, Kobayashi A, Shiro M, Ota N, Takano R, Mabuchi Y, Yagi S, Minami S, Terada M, Ino K. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int J Gynecol Cancer 2014;24:454-60. [Crossref] [PubMed]
- Konishi H, Takehara K, Kojima A, Okame S, Yamamoto Y, Shiroyama Y, Yokoyama T, Nogawa T, Sugawara Y. Maximum standardized uptake value of fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in ovarian clear cell adenocarcinoma. Int J Gynecol Cancer 2014;24:1190-4. [Crossref] [PubMed]
- Lee JW, Cho A, Lee JH, Yun M, Lee JD, Kim YT, Kang WJ. The role of metabolic tumor volume and total lesion glycolysis on (1)(8)F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur J Nucl Med Mol Imaging 2014;41:1898-906. [Crossref] [PubMed]
- Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, Kang SB, Kim JW. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol 2012;19:1966-72. [Crossref] [PubMed]
- Fanfani F, Monterossi G, Fagotti A, Gallotta V, Costantini B, Vizzielli G, Petrillo M, Carbone MV, Scambia G. Positron emission tomography-laparoscopy based method in the prediction of complete cytoreduction in platinum-sensitive recurrent ovarian cancer. Ann Surg Oncol 2015;22:649-54. [Crossref] [PubMed]
- Gu B, Xia L, Ge H, Liu S. Preoperative PET/CT score can predict complete resection in advanced epithelial ovarian cancer: a prospective study. Quant Imaging Med Surg 2020;10:743-53. [Crossref] [PubMed]
- Omura G, Blessing JA, Ehrlich CE, Miller A, Yordan E, Creasman WT, Homesley HD. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer 1986;57:1725-30. [Crossref] [PubMed]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1-6. [Crossref] [PubMed]
- Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, Ball H, Berek JS. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 1994;170:974-9; discussion 979-80. [Crossref] [PubMed]
- Buy JN, Moss AA, Ghossain MA, Sciot C, Malbec L, Vadrot D, Paniel BJ, Decroix Y. Peritoneal implants from ovarian tumors: CT findings. Radiology 1988;169:691-4. [Crossref] [PubMed]
- Guidozzi F, Sonnendecker EW. Evaluation of preoperative investigations in patients admitted for ovarian primary cytoreductive surgery. Gynecol Oncol 1991;40:244-7. [Crossref] [PubMed]
- Metser U, Jones C, Jacks LM, Bernardini MQ, Ferguson S. Identification and quantification of peritoneal metastases in patients with ovarian cancer with multidetector computed tomography: correlation with surgery and surgical outcome. Int J Gynecol Cancer 2011;21:1391-8. [Crossref] [PubMed]
- Simon A, Fields S, Schenker JG, Anteby SO. Computed tomography prior to surgery for ovarian carcinoma. Aust N Z J Obstet Gynaecol 1986;26:199-202. [Crossref] [PubMed]
- Hynninen J, Kemppainen J, Lavonius M, Virtanen J, Matomaki J, Oksa S, Carpen O, Grenman S, Seppanen M, Auranen A. A prospective comparison of integrated FDG-PET/contrast-enhanced CT and contrast-enhanced CT for pretreatment imaging of advanced epithelial ovarian cancer. Gynecol Oncol 2013;131:389-94. [Crossref] [PubMed]


