Vascular anomalies of the head and neck in children
Introduction
Vascular anomalies of the head and neck region constitute approximately 60% of vascular anomalies diagnosed in children and affect approximately 1 in 22 children (1,2). There is a wide spectrum of putative underlying causative factors. These lesions broadly fall into two categories—vascular tumours and vascular malformations—and both have physical and psychological implications for both the patient and their family, particularly if visibly disfiguring. The psychological impact should be considered at each stage of the child’s clinical management. Early assessment of specific clinical features including age at presentation, rate of growth, location and secondary local, systemic and psychological effects is essential to allow timely investigation and appropriate management.
Infantile haemangiomas (IHs) can usually be distinguished from other vascular tumours and malformations by their classic history and presentation, detailed below. In almost all IH cases the diagnosis can be made with history and examination alone and no further investigation or intervention is required.
For other lesions, imaging with ultrasound (US) and/or magnetic resonance imaging (MRI) offers exquisite soft tissue detail with delineation of blood vessel architecture and flow patterns. A diverse group of lesions presenting in complex anatomical locations, a multidisciplinary approach is vital with representation from specialists in paediatrics, dermatology, plastic surgery, orthopaedic and craniofacial surgery, otorhinolaryngology, oncology, neurosurgery, interventional radiology and neuro-radiology, as well as supportive input from psychology, physiotherapy and occupational therapy when needed.
Treatment options for vascular malformations include conservative management, drug treatment, minimally invasive interventions by interventional radiology, laser therapy and open surgery. A combination of several of these therapies is often required. Treatment is often multi-staged: aims and end-points of treatments must be clearly defined by the multidisciplinary team (MDT) at the outset and agreed with patients and/or their families.
Clinical presentation and assessment
Vascular anomalies of the head and neck usually present in childhood. They may be detected with antenatal imaging, present as a ‘birthmark’ in the neonate or emerge as a cutaneous lesion or palpable mass in later childhood. A full clinical history should be taken and examination performed in order to establish if the lesion is symptomatic, isolated or multiple, whether it is stable or evolving and the extent to which it involves local structures, particularly the airway, eye or other neural structures (Table 1).

Full table
The physician should exclude features of associated syndromes as a vascular lesion may herald more extensive underlying pathology. For example, widespread cutaneous telangiectasia is found in hereditary haemorrhagic telangiectasia (HHT) which is associated with arteriovenous malformations (AVMs) elsewhere in the brain, liver and lung. Patients with Sturge-Weber syndrome have a facial port wine stain (PWS) [a capillary malformation (CM)] and an associated intracranial pial angioma predisposing to seizures. The ‘PHACES’ syndrome (posterior fossa malformation, haemangioma of the face or neck, arterial stenosis, aneurysm or occlusion, cardiovascular anomalies, eye abnormalities, sternal cleft and supra-umbilical raphe) is used to describe patients with a variety of midline structural abnormalities and a characteristic flat, telangiectatic form of facial haemangioma.
At this stage the cosmetic and psychological impact of the lesion should be considered and relevant support arranged.
Classification
Haemangiomas and vascular malformations are endothelial disorders divided into two distinct pathological groups: vascular tumours (including haemangiomas) and vascular malformations (3). They were first dichotomised in this way by Mulliken and Glowacki in 1982, based on the natural history, histology and cellular activity of these lesions (4) (Table 2).

Full table
Vascular tumours are composed of rapidly proliferating cells and incomplete blood vessels (5); historically, descriptive terms such as ‘blood-containing lesions’, ‘angiomas’ or ‘birthmarks’ were used; and lesions were categorised as capillary, strawberry or cavernous based upon their component vessel size. This distinction is no longer considered clinically relevant and has been replaced by the now widely recognised International Society for the Study of Vascular Anomalies (ISSVA) classification of 2007, based on the original Mulliken and Glowacki dichotomous system (3).
IH makes up the large majority of lesions in the vascular tumours category and are described below.
Vascular malformations, by contrast, are present at birth and grow proportionately with the child. They are composed of dysplastic arterial, venous and/or lymphatic vessels rather than proliferating cells. Unlike IH spontaneous involution does not occur (6). They are named according to the predominant vessel type and are further classified as ‘high-flow’ and ‘low-flow’ lesions. Lesions that demonstrate arteriovenous shunting such as arteriovenous malformations (AVMs) and arteriovenous fistulae (AVF) are described as high flow, whereas venous malformations (VMs), lymphatic malformations (LMs) or combined lympho-venous/veno-lymphatic malformations (VLMs), together with CMs are described as low flow (7).
Differential diagnosis
Vascular tumours
Infantile haemangioma (IH)
Background and epidemiology
IHs are the most common tumours of infancy, affecting between 5% and 10% of the Caucasian population, with 60% being located in the head and neck (8). They are more common in Caucasian females with a female: male ratio of 2.4-4:1 (9) and can be associated with low birth weight, prematurity, multiple gestation and chorionic villus sampling (4). Lesions undergo a rapid proliferative period lasting months during young infancy, followed by a longer period of gradual involution throughout childhood. Up to 90% resolve completely by the age of 9 years (10).
They are principally composed of highly proliferative hyperplastic endothelial cells and the majority cause no clinical issue, require no investigation and can be left alone to involute with time. Some lesions of the head and neck cause disturbance of the visual axis or airway compromise due to mass effect and therefore require early intervention (Figure 1).

Presentation
IH typically present as a small lesion 2-6 weeks after birth. They undergo a period of rapid proliferation in the first 12-18 months of life followed by a phase of involution. Most appear as bright red exophytic lesions of the skin, though deep-seated lesions can cause no discolouration.
Up to 20% of IH are multiple—if more than five are present, this can indicate internal haemangiomatosis. The liver is the most common site of extra-cutaneous lesions and hepatic haemangiomas are associated with high output cardiac failure that can threaten life. If more than five IHs are found, therefore, imaging with liver US should be arranged (11).
Management
For simple lesions conservative management following a comprehensive clinical history and examination at initial presentation is sufficient. In approximately 10% of patients IH can be complicated, particularly those involving the airway, the oral cavity or the eye (1,12,13). In this situation, imaging with US or MRI and referral to a specialist should be arranged.
Definitive management of almost all complicated IHs is with oral propranolol alone (14). Propranolol has replaced oral corticosteroids as a first line treatment for IH and has been shown in multiple studies to be safe and effective in reducing the size and discolouration of IH, possibly by induction of vasoconstriction (15). More complicated lesions can be managed surgically with laser treatment or resection, although surgical therapy is limited by lesion vascularity and a combined approach is often adopted (16).
Prognosis
In uncomplicated lesions, prognosis is excellent with 90% resolution by 9 years of age and a low incidence of scarring.
For complicated lesions prognosis is variable, with considerable morbidity but low mortality largely associated with lesions in certain locations or underlying syndromes such as PHACES syndrome (17) (Figure 2).

Congenital haemangioma (CH)
Background and epidemiology
In contrast to IH, CHs are rare. They are fully formed at birth.
CHs are classified into two types depending on their clinical behaviour: rapidly involuting congenital haemangioma (RICH) and non-involuting congenital haemangioma (NICH). Histologically both differ from IH in that they lack glucose transporter-1 protein expression. They differ from each other in component vessel size, endothelial cell and pericyte size, although there is some overlap between the two (18,19).
RICH begin their rapid involutional phase soon after birth and usually resolve by 1 year of age, whereas NICH never involute. Like all haemangiomas these lesions are highly vascular. RICHs can be very large at birth and cause high output cardiac failure in the neonatal period because of significant blood flow to the lesion.
Presentation
Patients present at birth following antenatal suspicion or observation of a cutaneous lesion postpartum. RICHs are round or oval, raised and sometimes infiltrating vascular lesions that can be ulcerated at presentation. NICHs present as a warm, pink to blue/violet mass associated with central, coarse telangiectasia—occasionally peripheral draining veins are seen.
Investigation
If involution is not observed after a period of 4-6 months further assessment with imaging may be indicated, although conservative management is preferred where NICH is suspected. Primary investigation is usually by US and can demonstrate a low resistance, variable flow lesion of varying echogenicity during the proliferative phase.
Contrast enhanced MRI (CE-MRI) will demonstrate a homogeneously enhancing, vascular, solid mass. This also allows assessment of lesion extent and mass effect on surrounding structures (Figure 3).

Digital subtraction angiography (DSA) is reserved for patients undergoing endovascular treatment because of the invasive nature of the examination and should be arranged only after MDT discussion and performed in a specialist centre with experience in paediatric cerebral angiography.
Management
Conservative management with a ‘watch-and-wait’ approach is adopted in most cases. Those complicated by haemorrhage, ulceration, heart failure, airway obstruction or ophthalmic involvement and those causing severe psychological distress or disfigurement warrant further discussion and consideration for treatment
RICH presenting with acute cardiac failure may require emergency embolisation to reduce arteriovenous shunting. NICH can be surgically treated but are usually small and do not require treatment.
Prognosis
The majority of RICH resolve by 1 year of age.
Kaposiform hemangioendothelioma (KH)
KH is a rare, locally aggressive but benign lesion that is usually seen in infants and children. It is locally invasive, but has very low malignant potential. KH is associated with Kasabach-Meritt phenomenon, a term which describes a severe coagulopathy due to massive consumption of platelets in the lesion vascular bed causing a profound thrombocytopaenia (20). KH lesions typically present as isolated or multiple purplish, purpuric macules which can macroscopically resemble IH or RICH (21,22) (Figure 4).

Tufted angioma (TA)
Also associated with Kasabach-Meritt phenomenon, TA is most often seen in the neck and upper body of children younger than 5 years (23). The lesion presents as a red, maculopapular plaque. TAs are slow growing, benign lesions but can be very painful and cause contractures resulting in major disfigurement. They are not known to have malignant potential but are sometimes confused with malignant lesions (Kaposi’s sarcoma or angiosarcoma), clinically (24).
Vascular malformations: low flow
Capillary malformations (CMs)
CMs are low flow, sporadic, congenital, persisting post-capillary venule malformations that affect the head or neck. Most are asymptomatic but can be treated with laser therapy to decrease skin thickening and discolouration.
Port Wine Stains (PWSs)
Background and epidemiology
PWSs are an important subtype of CM that affect less than 1% of children with no sex predominance (25). These lesions should be differentiated from simple CM because of important associations with conditions such as Sturge-Weber syndrome and Klippel-Trenaunay syndrome (26).
Presentation
PWS present at birth, growing and darkening in colour with age, and are associated with varying degrees of disfigurement as a result of local soft tissue and bony hypertrophy. They are unilateral in 90% and commonly affect the cutaneous distribution of the ophthalmic and maxillary trigeminal nerve divisions, although lesions of the mandibular division are also seen.
Where a facial PWS is present, an underlying diagnosis of Sturge-Weber syndrome should be considered. In this syndrome the skin lesion is associated with a pial angioma, a vascular malformation over the surface of a region of the brain, which may cause seizures, cerebral hypometabolism and intracranial haemorrhage. This association is seen in up to one in four children with a unilateral ophthalmic-distribution PWS, and in up to one in three children where the lesion is bilateral. There is no association of Sturge-Weber syndrome with lesions of the maxillary or mandibular regions of the face (27).
Investigation
Although the cutaneous lesion can be diagnosed clinically, in patients with ophthalmic lesions a paediatric neurologist should arrange CE-MRI of the brain as early diagnosis and management of a pial angioma can minimise subsequent seizure activity and resultant brain injury (28) (Figure 5).

Management
Referral to a vascular anomaly MDT is recommended with a view to arranging appropriate psychological, medical and potentially surgical support. In ophthalmic lesions, the eye of the affected side is at risk of glaucoma and therefore paediatric ophthalmologists should be consulted.
The psychological impact of a disfiguring facial lesion is the major rationale for active management, primarily by laser photocoagulation. Several treatments can be required and the lesion might never be cleared, although prognosis is improved if treatment is instigated at less than 6 months of age (because of the lesions’ smaller size and reduced disruption to the underlying tissues) (29). Surgery for debulking, reconstructive flaps or for haemostasis of bleeding nodules is sometimes indicated.
Prognosis
PWSs are persistent and can cause severe disfigurement if untreated. Apart from complications of glaucoma and underlying intracranial disease in Sturge-Weber syndrome, however, PWS follow an otherwise benign clinical course.
Venous malformations (VMs)
Background and epidemiology
VMs constitute up to 40% of all diagnosed vascular anomalies (30). They affect males and females equally like all malformations that are present at birth.
Unlike IH, they grow with the child and do not spontaneously involute, typically becoming larger around puberty, when they may require intervention. VMs can involve soft tissue and bone and can cause disfigurement or disability.
Presentation
Children usually present with a bluish, fluctuant, soft skin lesion on either the cutaneous skin or mucous membranous surface, although deep-seated lesions may not cause discolouration. The lesion can swell (particularly in the morning because of stasis of flow overnight) but chronic pain is a more common feature secondary to mass effect, venous congestion and intermittent painful thrombosis. There is no palpable thrill or audible bruit on clinical examination. The lesions are soft and can be compressed (or ‘emptied’). They change with patient position and Valsalva manoeuvres as they are flow dependent.
Investigation
Full clinical assessment of the extent of the lesion and associated deformity must be undertaken. VMs comprise abnormal vessels lined with abnormal endothelium and can be associated with coagulopathy and elevated D-Dimer levels (31).
The imaging investigation of choice is US which may show characteristic phleboliths (well defined, round calcific lesions) representing areas of spontaneous thrombosis. Doppler US will demonstrate slow flow in compressible, dilated vascular channels.
CE-MRI provides anatomical assessment of lesion extent and complications such as bone or orbital involvement (Figure 6).
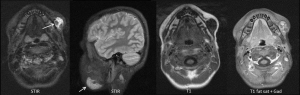
Management
The majority of lesions are small and relatively asymptomatic, requiring no intervention. Compression of larger extremity lesions with graded compression garments can help to alleviate swelling and pain. Medical management remains controversial but early studies have shown some benefit from long-term low molecular weight heparin (32) and anti-platelet drugs (33) in provision of some symptomatic relief and possible prevention of localised intravascular coagulopathy and thrombosis.
Percutaneous sclerotherapy is generally regarded as the mainstay of treatment or can form part of a combined approach, together with surgery. Most patients require multistage treatment and sclerotherapy is used to manage symptoms, not as a cure.
Surgical resection can be definitive in well-defined lesions. However, it is usually complex and complete resection is often precluded by the diffuse nature of most VMs. Recurrence rates are high.
Prognosis
Treatment is targeted towards prevention of progression and symptomatic relief rather than cure for most VM patients. Even when surgery is undertaken recurrence rates are high. Misdiagnosis as IH can lead to mismanagement and catastrophic surgical complications and so MDT-centred care is critical for these patients.
Lymphatic malformations (LMs)
Background and epidemiology
LMs affect the sexes equally. They are sub-classified depending upon the size of the lymphatic spaces. In general, if the cysts are ≥1 cm in diameter the lesions are macrocystic; whereas channels of ≤1 cm constitute a microcystic lesion. Lesions can be mixed.
Presentation
LM present at birth in up to 60% of patients and by the age of 2 years in approximately 90%. Lesions can be confined to the skin or can infiltrate and disrupt local anatomical spaces, viscera and bone or soft tissues. Clinical presentation can therefore be diverse and include airway obstruction, neurovascular dysfunction and deformity.
Investigation and management
For LM this is comparable to that of VM and demands thorough clinical assessment and referral to a vascular anomaly MDT.
Imaging with US is performed to classify lesion components and their channel size, to assess anatomical extent of the lesion and to plan treatment. MRI of macrocystic lesions will show a non-enhancing, thin walled, uni- or multi-loculated cystic lesion with septal enhancement (Figure 7). Management should be strictly MDT-led. Management of symptomatic lesions involves surgery and/or sclerotherapy.

Surgical resection has a larger role in the treatment of LM than in VM. Pre-operative image-based staging of lesions is performed to help predict risk and outcome as surgery can be complex (34).
Prognosis
As for VM, treatment for LM is largely symptomatic rather than curative. These lesions can be difficult to treat (35) and MDT involvement is crucial.
Vascular malformations: high flow
Arteriovenous malformations (AVMs)
Background and epidemiology
AVMs are rare, congenital, dynamic vascular lesions. A nidus forms a direct communication between the arterial and venous systems (in the absence of a normal capillary bed) resulting in shunting of blood under arterial pressure into the low pressure venous system (36).
Presentation
Many AVMs are small and asymptomatic, although a proportion shows progression in adolescence.
Growth and local invasion can cause disfigurement, functional disturbance, haemorrhage and high output cardiac failure (37). Clinical progression is staged by the Schöbinger classification (Table 3) (38).

Full table
Investigation
Referral to the MDT with input from an interventional neuroradiologist is preferable for suspected high flow lesions of the head and neck. Most can be diagnosed and adequately assessed by clinical examination although Doppler US can be used to confirm the presence of AV shunting and initial investigation typically includes DSA performed by an interventional neuroradiologist. MRI of the head and neck can be performed to assess the lesion’s extent and intracranial involvement.
Suitability for diagnostic DSA and endovascular embolisation should be decided by the MDT and the performing interventional neuroradiologist.
Management
Treatment is centred on obliteration of the nidus which can be achieved percutaneously, endovascularly or surgically (Figure 8).

Prognosis
AVMs are a highly heterogeneous group of lesions and general predictions over outcome cannot be made: small lesions may never enlarge or progress beyond Schöbinger stage I and, as such, can be left alone. A proportion of symptomatic lesions can be adequately treated by embolization and then remain quiescent through life. Some, however, prove difficult to manage and require repeated intervention. More complex lesions can result in life threatening complications.
Vascular malformations: mixed lesions
Any combination of venous, lymphatic and arterial components can be seen within complex vascular malformations (3,30,32,34,35).
Conclusions and main messages
- Not all vascular anomalies of the head and neck are haemangiomas. Accurate early clinical assessment can be of great benefit to clinical outcome.
- Lesions are classified into vascular tumours and vascular malformations according to their natural history and histological components.
- Vascular anomalies of the head and neck can cause disfigurement and disability and have a severe psychological impact on patients and their families.
- Where there is any clinical doubt about diagnosis of vascular anomalies, referral to a specialist centre is mandatory.
- Imaging, where required, usually includes US and MRI.
- The majority of lesions can be managed conservatively. A small proportion requires medical, endovascular or surgical intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hemangioma Investigator Group. Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr 2007;150:291-4. [PubMed]
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med 1999;341:173-81. [PubMed]
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Odile Enjolras, Michel Wassef, Rene Chapot, editors. Color Atlas of Vascular Tumors and Vascular Malformations. New York: Cambridge University Press, 2007.
- Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412-22. [PubMed]
- Nozaki T, Matsusako M, Mimura H, Osuga K, Matsui M, Eto H, Ohtake N, Manabe A, Kusakawa I, Tsutsumi Y, Nosaka S, Kamo M, Saida Y. Imaging of vascular tumors with an emphasis on ISSVA classification. Jpn J Radiol 2013;31:775-85. [PubMed]
- Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis 2010;16:405-18. [PubMed]
- Jackson IT, Carreño R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg 1993;91:1216-30. [PubMed]
- Léauté-Labrèze C, Taïeb A. Efficacy of beta-blockers in infantile capillary haemangiomas: the physiopathological significance and therapeutic consequences. Ann Dermatol Venereol 2008;135:860-2. [PubMed]
- Drolet BA, Swanson EA, Frieden IJ; Hemangioma Investigator Group. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr 2008;153:712-5, 715.e1.
- Starkey E, Shahidullah H. Propranolol for infantile haemangiomas: a review. Arch Dis Child 2011;96:890-3. [PubMed]
- Nord KM, Kandel J, Lefkowitch JH, Lobritto SJ, Morel KD, North PE, Garzon MC. Multiple cutaneous infantile hemangiomas associated with hepatic angiosarcoma: case report and review of the literature. Pediatrics 2006;118:e907-13. [PubMed]
- Broeks IJ, Hermans DJ, Dassel AC, van der Vleuten CJ, van Beynum IM. Propranolol treatment in life-threatening airway hemangiomas: a case series and review of literature. Int J Pediatr Otorhinolaryngol 2013;77:1791-800. [PubMed]
- Hernandez JA, Chia A, Quah BL, Seah LL. Periocular capillary hemangioma: management practices in recent years. Clin Ophthalmol 2013;7:1227-32. [PubMed]
- Hartzell LD, Buckmiller LM. Current management of infantile hemangiomas and their common associated conditions. Otolaryngol Clin North Am 2012;45:545-56. vii. [PubMed]
- Shayan YR, Prendiville JS, Goldman RD. Use of propranolol in treating hemangiomas. Can Fam Physician 2011;57:302-3. [PubMed]
- Frieden IJ, Haggstrom AN, Drolet BA, Mancini AJ, Friedlander SF, Boon L, Chamlin SL, Baselga E, Garzon MC, Nopper AJ, Siegel DH, Mathes EW, Goddard DS, Bischoff J, North PE, Esterly NB. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol 2005;22:383-406. [PubMed]
- Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics 2006;118:882-7. [PubMed]
- Krol A, MacArthur CJ. Congenital hemangiomas: rapidly involuting and noninvoluting congenital hemangiomas. Arch Facial Plast Surg 2005;7:307-11. [PubMed]
- Berenguer B, Mulliken JB, Enjolras O, Boon LM, Wassef M, Josset P, Burrows PE, Perez-Atayde AR, Kozakewich HP. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol 2003;6:495-510. [PubMed]
- Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg 1997;100:1377-86. [PubMed]
- Chang JM, Kwon BJ, Han MH, Kang HS, Chang KH. Kaposiform hemangioendothelioma arising from the internal auditory canal. AJNR Am J Neuroradiol 2006;27:931-3. [PubMed]
- Mac-Moune Lai F, To KF, Choi PC, Leung PC, Kumta SM, Yuen PP, Lam WY, Cheung AN, Allen PW. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol 2001;14:1087-92. [PubMed]
- Jones EW, Orkin M. Tufted angioma (angioblastoma). A benign progressive angioma, not to be confused with Kaposi's sarcoma or low-grade angiosarcoma. J Am Acad Dermatol 1989;20:214-25. [PubMed]
- Wilmer A, Kaatz M, Bocker T, Wollina U. Tufted angioma. Eur J Dermatol 1999;9:51-3. [PubMed]
- Sidoroff A. Epidemiology of cutaneous vascular neoplasms and malformations in childhood. Handchir Mikrochir Plast Chir 2009;41:65-9. [PubMed]
- Burns AJ, Navarro JA, Cooner RD. Classification of vascular anomalies and the comprehensive treatment of hemangiomas. Plast Reconstr Surg 2009;124:69e-81e. [PubMed]
- Hochman M, Adams DM, Reeves TD. Current knowledge and management of vascular anomalies, II: malformations. Arch Facial Plast Surg 2011;13:425-33. [PubMed]
- Juhasz C, Batista CE, Chugani DC, Muzik O, Chugani HT. Evolution of cortical metabolic abnormalities and their clinical correlates in Sturge-Weber syndrome. Eur J Paediatr Neurol 2007;11:277-84. [PubMed]
- Chapas AM, Eickhorst K, Geronemus RG. Efficacy of early treatment of facial port wine stains in newborns: a review of 49 cases. Lasers Surg Med 2007;39:563-8. [PubMed]
- Greene AK. Vascular anomalies: current overview of the field. Clin Plast Surg 2011;38:1-5. [PubMed]
- Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, Deneys V, Pocock B, Lequerrec A, Labbé D, Barrellier MT, Vanwijck R, Vikkula M, Boon LM. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol 2008;144:873-7. [PubMed]
- Dompmartin A, Vikkula M, Boon LM. Venous malformation: update on aetiopathogenesis, diagnosis and management. Phlebology 2010;25:224-35. [PubMed]
- Hein KD, Mulliken JB, Kozakewich HP, Upton J, Burrows PE. Venous malformations of skeletal muscle. Plast Reconstr Surg 2002;110:1625-35. [PubMed]
- de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg 1995;121:577-82. [PubMed]
- Balakrishnan K, Edwards TC, Perkins JA. Functional and symptom impacts of pediatric head and neck lymphatic malformations: developing a patient-derived instrument. Otolaryngol Head Neck Surg 2012;147:925-31. [PubMed]
- Young AE, Mulliken JB. Arteriovenous malformations. In: JB Mulliken, AE Young, editors. Vascular Birthmarks: Haemangiomas and Malformations. Philadelphia: Philadelphia WB Saunders, 1988:228-45.
- Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg 2010;125:1185-94. [PubMed]
- Schöbinger R. In: Proceedings of International Society for the Study of Vascular Anomalies Congress. Rome, Italy, 1996.


