
Imaging features of mycotic aortic aneurysms
Introduction
Infectious aortitis (IA) is a rare, life-threatening cardiovascular disease for which early diagnosis can be missed due to a lack of specific clinical, radiological, and laboratory features. IA’s common sign is a mycotic aortic aneurysm (MAA), which manifests as a mushroom-shaped structure on a blood vessel. This manifestation does not refer to a specific pathogenic cause, such as a fungal infection (1); rather, MAAs is an acute inflammatory response to pathogenic infection, which induces neutrophilic infiltration at the arterial wall. During this process, the collagenolytic and elastolytic enzymes are activated, which is concomitant with saccular lumen dilation and rupture (2-4).
MAAs are associated with high mortality due to their increased risk of rupture, which is especially common in the abdominal aorta compared with peripheral arteries (5). Early diagnosis and timely intervention are critical in reducing the mortality of MAAs; however, early diagnosis is challenging due to nonspecific symptoms and low sensitivity of blood cultures (6-9). Furthermore, the incidence of adverse events in patients with MAAs after invasive treatment is higher than those with pseudoaneurysms associated with other causes, such as trauma and atherosclerosis. The endovascular stenting of MAAs may be associated with a high risk of stent infection, endoleak, reinfection, and potential rupture (10,11).
The diagnosis of MAAs requires awareness of the spectra of computed tomography (CT), magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT. The use of CT angiography (CTA) in the assessment of aortic disease—including MAAs—is increasing, owing to its non-invasive, efficient, broad coverage and its isotropic voxel capabilities. With CT technology development and advanced dose reduction techniques, CTA is a fast and high-quality method with minimal contrast medium and radiation dose. The high tissue resolution of MRI can provide valuable anatomical and physiological information, especially in assessing abscess and tissue edema. In the latest studies, 18F-FDG-PET/CT has shown higher sensitivity and diagnostic accuracy in infected aortic aneurysms and aortic prosthetic graft infection compared to CTA (12,13).
This review provides an overview of the clinical, pathological, and radiological presentations of MAAs. The imaging findings during the period following medication, interventional, or surgical management are also described. The imaging characteristics in other infectious pathogens are highlighted for differential diagnosis, especially in patients with negative blood culture results.
Pathogenesis
MAAs usually occur in the elderly, predominantly affecting immunocompromised patients, such as diabetes mellitus, liver cirrhosis, end-stage renal disease, alcoholism, chronic glucocorticoid therapy, post-transplantation immunosuppression, human immunodeficiency virus infection, drug abuse, and malignancy (14-17). The known causative organisms of MAAs are Salmonella, Staphylococcus aureus, Klebsiella pneumoniae (KP), Escherichia coli, Mycobacterium, and Brucella melitensis. Fungi, such as Candida albicans and Aspergillus, are also rare causes of infected aneurysms.
Salmonella
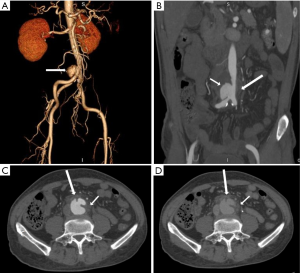
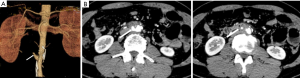
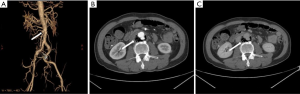
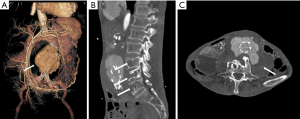
Non-typhoidal Salmonella has been reported as the most common organism in Eastern countries, especially in the atherosclerotic abdominal aorta (8,16,18-20). Salmonella usually resides in the phagosomes of host macrophages and other antigen-presenting cells, such as dendritic cells, which participate in the formation of atherosclerotic plaques. The immunocompromised condition contributes to its reproduction and invasiveness from atherosclerotic plaques (21-23) (Figure 1).
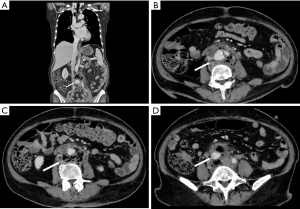
Klebsiella pneumoniae (KP)
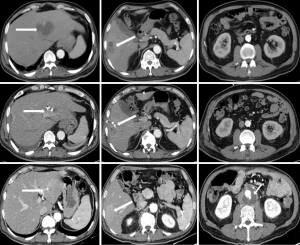
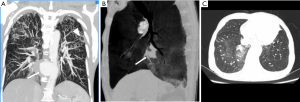
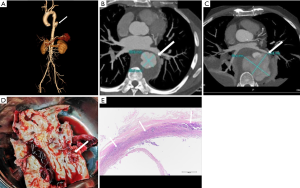
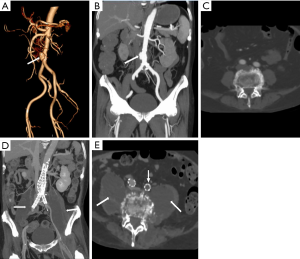
Klebsiella infection can occur in almost all organs but is most commonly observed in the liver and lungs. Approximately 60–93% of patients with KP have comorbid diabetes mellitus (15,24,25). KP can invade the aortic wall and induce MAAs from the location of damaged vascular endothelial walls (16). In patients suffering from aortic pseudoaneurysm and pyogenic liver abscess and who have a history of diabetes mellitus, KP should be considered as the causative organism (Figure 2).
Mycobacterium tuberculosis
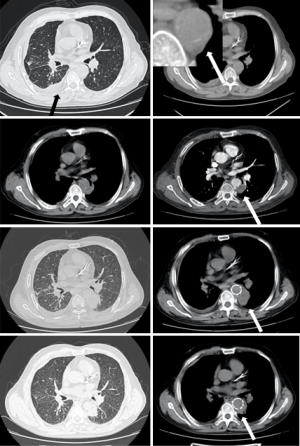
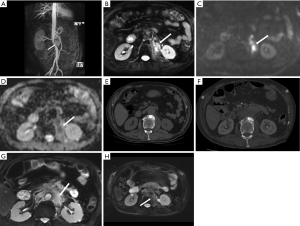
About 75% of MAAs caused by Mycobacterium tuberculosis present as a contiguous lesion on the surrounding tissue, such as lymph node enlargement or paraspinal abscess (26,27). Constant surveillance imaging can indicate pathogenesis (Figure 3).
Staphylococcus aureus
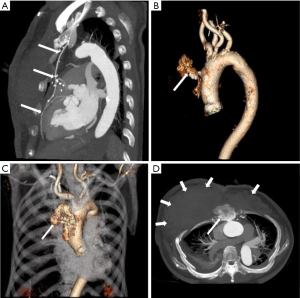
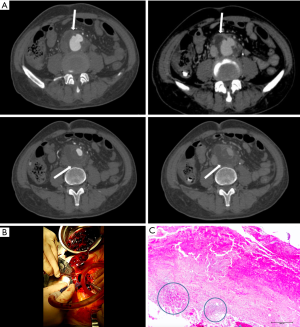
Pseudoaneurysm caused by Staphylococcus aureus has been reported in intravenous drug users, as well as iatrogenic or traumatic arterial wall injury patients (28,29) (Figure 4). In these patients, MAAs develop from direct infectious inoculation at the time of vascular trauma.
Brucella melitensis
Brucella melitensis is a zoonotic intracellular Gram-negative coccobacillus responsible for multisystem infections, including the aorta—especially in immunocompromised patients. The common transmission route is through direct contact with infected cattle via milk or other body fluids (30).
Clinical features
Due to the risk of rapid expansion and consequent rupture, timely diagnosis and treatment of MAAs are paramount. Unfortunately, some patients could be clinically silent until an aneurysm rupture. The most frequent presenting symptoms of MAAs are fever and pain (16,19). MAAs localized to the thoracic aorta usually manifest as chest pain, whereas infected abdominal aortic aneurysms usually manifest as abdominal pain with or without a pulsatile mass. The laboratory abnormalities are often nonspecific and may include elevated erythrocyte sedimentation rate (ECR), C-reactive protein (CRP), and leukocytosis. Blood cultures fail to detect bacteria in approximately 25% of cases, which might contribute to broad-spectrum antibiotic therapy administration.
Life-threatening hemorrhage—such as hemoptysis, gastrointestinal hemorrhage, and sequentially shock—could result from fistulae. The poor prognosis of these patients emphasizes the importance of early diagnosis. Aortoenteric fistula is caused by aneurysm infection spreading to the enteron, which usually begins at the duodenum, adjacent to the aorta (Figure 5). Thoracic MAAs with surrounding lung parenchyma infection or compaction cause aortobronchial fistula (Figure 6), a less recognized complication of abdominal MAAs. A dramatic increase in mortality occurs in patients without clear preoperative diagnosis compared to those with clear preoperative diagnosis (100% in patients without a clear diagnosis vs. 15% in patients with clear diagnosis).
Iliopsoas abscess (IPA) is a common complication in abdominal MAAs with or without endograft infection, presenting as a direct invasive infection with purulent materials occurring within the iliopsoas muscle. The causative organisms include Mycobacterium, Salmonella, KP, other Gram-negative bacilli, and mixed bacteria. The mortality rate is as high as 100% if IPA is left untreated (31-33). IPA is reported as a major risk factor for patients with MAAs; thus, clinicians should be cautious about this potential complication. In tuberculous spondylitis patients, MAAs can involve secondary spread from spine lesions (Figure 7). The rate of mortality is high among MAA patients with combined pyogenic spondylitis (34,35).
Clinical management
Open surgical repair has been regarded as an effective treatment for MAA but is associated with a mortality rate of over 20% (17,29). The endovascular repair of MAAs—an expeditious temporization of MAA rupture in hemodynamic instability cases—is well-established. Kan et al. demonstrated that there was no significant difference in overall survival rate between open surgical repair and endovascular repair (20); however, the insertion of an endovascular graft in an infected field remains a major concern. Stent graft implantation is a significant independent predictor of persistent infection (20). Prolonged culture-specific antibiotic therapy, combined with open surgery or endovascular techniques, is a key component for successfully treating MAAs.
Imaging features
CT, MRI, and PET/CT are the most commonly used imaging modalities in the detection and assessment of clinically suspected infected aneurysms. Due to the higher quality spatial resolution of contrast-enhanced CT and MRI, these modalities can provide valuable information regarding the morphology of aortic aneurysm, aortic wall enhancement, and the relationship between the aneurysm and adjacent tissue; however, PET/CT is the most sensitive of these modalities in detecting infection, which is depicted by increased uptake of FDG. Like PET/CT, diffusion-weighted imaging (DWI) MRI is also sensitive in detecting infection with restricted diffusion manifestation. DWI and T2-weighted MRI can assist in detecting soft tissue edema and adjacent organ involvement.
Infected aortic aneurysm
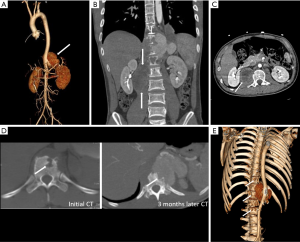
An infected aortic aneurysm appears on CT and MRI as a focal, contrast-enhancing, lobulated, saccular lumen, with an indistinct, irregular aortic wall (Figure 8). CT is the most sensitive imaging modality for detecting calcification and gas bubbles. The calcification interruption indicates the location of the disrupted aortic wall. Gas bubbles that appear in and around MAAs have high diagnostic reliability of etiology (Figure 9). Rapidly progressive growth of true or false aneurysms (>5 mm in 2 weeks) is also suggestive of an infectious etiology (Figure 10). The thickened MAA wall usually appears as a high signal intensity on T2-weighted MRI and DWI (Figure 11), with increased uptake of FDG on PET/CT.
Periaortic tissue
Eccentric periaortic inflammatory soft tissue usually manifests as rim or septum enhancement following the administration of contrast material (venous phase) on contrast-enhanced CT and MRI (Figure 8). Periaortic edema appears as a distinctive fat stranding on CT; lymph nodes adjacent to MAAs might also appear swollen and enhanced (Figure 1). The edema of periaortic tissue and lymph nodes have the same MRI and PET/CT findings as the thickened MAAs wall described above (Figure 11).
Adjacent organs
CT provides a definitive diagnosis of IPA and other features that involve adjacent structures. IPA's typical features on CT include enlarged iliopsoas muscle, single or multiple relatively low-density abscess cavities with rim-like contrast-enhanced walls (Figure 7), and in some cases, gas within lesions. Interestingly, MRI and PET/CT are sensitive modalities in detecting IPA with typical restricted-diffusion manifestation on MRI and increased uptake of FDG on PET/CT (Figure 11).
Primary and secondary pyogenic spondylitis manifests intervertebral disc and/or vertebral body destruction on CT and MRI (Figure 12). The diagnosis of pyogenic spondylitis can also be confirmed on PET/CT with increased metabolic manifestations. MRI and PET/CT enable spondylitis visualization without morphological changes in the early stage (Figure 11).
Contrast medium shunt from the aorta to the inferior vena cava, digestive tract, or bronchus, is a diagnostic sign for aortic fistula (36,37). The shunt usually manifests as early enhancement of the inferior vena cava well before it appears in the renal and hepatic parenchyma, with a density of the adjacent digestive tract or bronchus similar to that of the adjacent aorta. When aortacaval fistula occurs, dilated and retrograded enhanced renal or iliac veins can be seen on enhanced CT and MRI, with direct communication between the aorta and inferior vena cava (Figure 13). Aortocutaneous fistulas are extremely rare and are usually seen in cases involving prior vascular prosthetic graft insertion (Figure 14) (38). Abnormal accumulation of FDG has diagnostic significance for aortoenteric and aortobronchial fistulae.
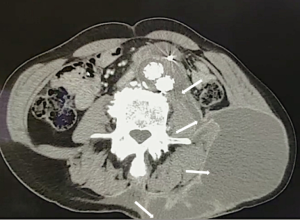
Follow-up imaging
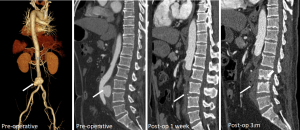
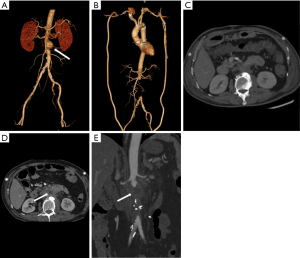
Recognition of infection-related complications should be considered during the analysis of follow-up images. Radiological signs are important diagnostic criteria in the detection of aortic graft infection (39), including ectopic gas, peri-graft inflammation, and fluid, thickening of adjacent bowel, pseudoaneurysm formation, and increased 18F-FDG uptake at the graft anastomosis (Figure 15) (13,39). The peri-graft fluid within the first 3 months after surgery may contribute to postoperative changes (39). Considering the white blood cell count and clinical symptoms, persistent existence, or gradual increase in the peri-graft fluid should be suspected as an infection-related complication. Extraanatomic bypass procedure avoids graft placement within an infected field, lowering the risk of graft reinfection compared to in situ reconstruction with a prosthetic graft (17). The endovascular repair of MAAs should be regarded as a temporizing treatment mainly performed on hemodynamically unstable patients. Compared to surgery, the challenges of wide debridement and effective drainage of suppuration brings risks of stent infection (Figure 16), endoleak (Figure 17), aortic fistulas, and potential rupture (8) (Figure 18). Spondylitis and IPA secondary to reinfection occur after both open surgery and endovascular repair (Figures 10,19). A prospective study—including 35 patients with suspected aortic graft infection—showed high concordance between 18F-FDG-PET/CT and expert consensus criteria from the Management of Aortic Graft Infection Collaboration (MAGIC) for detecting aortic graft infection (13). Further, 18F-FDG-PET/CT can also be used to monitor the response of MAAs and aortic graft infection to antibiotic treatment.
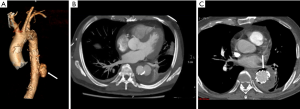

Conclusions
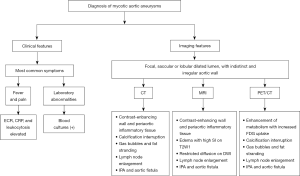
Diagnosis of life-threatening MAAs is challenging due to the non-specific symptoms and negative blood cultures associated with prior antibiotic use. CT, MRI, and FDG-PET/CT allow early identification of MAAs, which is crucial for improving patient outcomes. Surveillance imaging permits assessment and promotes treatment efficacy. Figure 20 summarizes both clinical and radiological features that can assist in the diagnosis of MAAs. Clinicians—especially cardiologists, vascular surgeons, and radiologists—should be familiar with the clinical features and common manifestations of MAAs to ensure appropriate evaluation and management of the disease.
Acknowledgments
The authors would like to thank Drs. Fengqiang Wang, Huaiping Yuan and Jin Cheng for their assistance in providing some cases that were used in this study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-941). Dr. ZS serves as an unpaid associate editor of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J 1885;1:522-6. [Crossref] [PubMed]
- Maleszewski JJ. Inflammatory ascending aortic disease: perspectives from pathology. J Thorac Cardiovasc Surg 2015;149:S176-83. [Crossref] [PubMed]
- Stone JR, Bruneval P, Angelini A, Bartoloni G, Basso C, Batoroeva L, Buja LM, Butany J, d’Amati G, Falloon JT, Gittenberger-de Groot AC, Gouveia RH, Halushka MK, Kelly KL, Kholova I, Leone O, Litovsku SH, Maleszewski JJ, Miller DV, Mitchell RN, Preston SD, Pucci A, Radio SJ, Rodriguez ER, Sheppard MN, Suvarna SK, Tan CD, Thiene G, van der Wal AC, Veinot JP. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol 2015;24:267-78. [Crossref] [PubMed]
- Buckmaster MJ, Curci JA, Murray PR, Liao S, Allen BT, Sicard GA, Thompson RW. Source of elastin-degrading enzymes in mycotic aortic aneurysms: bacteria or host inflammatory response? Cardiovasc Surg 1999;7:16-26. [Crossref] [PubMed]
- McCready RA, Bryant MA, Divelbiss JL, Chess BA, Chitwood RW, Paget DS. Arterial infections in the new millenium: an old problem revisited. Ann Vasc Surg 2006;20:590-5. [Crossref] [PubMed]
- Carreras M, Larena JA, Tabernero G, Langara E, Pena JM. Evolution of Salmonella aortitis towards the formation of abdominal aneurysm. Eur Radiol 1997;7:54-6. [Crossref] [PubMed]
- Maeda H, Umezawa H, Goshima M, Hattori T, Nakamura T, Umeda T, Shiono M. Primary infected abdominal aortic aneurysm: surgical procedures, early mortality rates, and a survey of the prevalence of infectious organisms over a 30-year period. Surg Today 2011;41:346-51. [Crossref] [PubMed]
- Plotkin A, Magee GA, Elsayed RS, Byerly S, Ham SW, Han SM, Manzur MF, Rowe VL, Weaver FA. Methicillin-resistant Staphylococcus aureus portends a poor prognosis after endovascular repair of mycotic aortic aneurysms and aortic graft infections. J Vasc Surg 2020;72:276-85. [Crossref] [PubMed]
- Sörelius K, di Summa PG. On the Diagnosis of mycotic aortic aneurysms. Clin Med Insights Cardiol 2018;12:1179546818759678 [Crossref] [PubMed]
- Jones KG, Bell RE, Sabharwal T, Aukett M, Reidy JF, Taylor PR. Treatment of mycotic aortic aneurysms with endoluminal grafts. Eur J Vasc Endovasc Surg 2005;29:139-44. [Crossref] [PubMed]
- Patel HJ, Williams DM, Upchurch GR Jr, Dasika NL, Eliason JL, Deeb GM. Thoracic aortic endovascular repair for mycotic aneurysms and fistulas. J Vasc Surg 2010;52:37S-40S. [Crossref] [PubMed]
- Husmann L, Huellner MW, Ledergerber B, Eberhard N, Kaelin MB, Anagnostopoulos A, Kudura K, Burger IA, Mestres CA, Rancic Z, Hasse B. Vasgra Cohort. Diagnostic accuracy of PET/CT and contrast enhanced CT in patients with suspected infected aortic aneurysms. Eur J Vasc Endovasc Surg 2020;59:972-81. [Crossref] [PubMed]
- Dong W, Li Y, Zhu J, Xia J, He L, Yun M, Jiao J, Zhu G, Hacker M, Wei Y, Zhang X, Li X. Detection of aortic prosthetic graft infection with 18F-FDG PET/CT imaging, concordance with consensus MAGIC graft infection criteria. J Nucl Cardiol 2020; [Crossref] [PubMed]
- Silvestri V, Ettorre GD, Borrazzo C, Mele R. Many Different Patterns under a Common Flag: Aortic Pathology in HIV-A Review of Case Reports in Literature. Ann Vasc Surg 2019;59:268-84. [Crossref] [PubMed]
- Pillsbury MM, Geha RM, Edson RS. Sticky Business: a syndrome of mucoid bacterial spread. BMJ Case Rep 2019;12:e226956 [Crossref] [PubMed]
- Luo Y, Zhu J, Dai X, Fan H, Feng Z, Zhang Y, Hu F. Endovascular treatment of primary mycotic aortic aneurysms: a 7-year single-center experience. J Int Med Res 2018;46:3903-9. [Crossref] [PubMed]
- Kyriakides C, Kan Y, Kerle M, Cheshire NJ, Mansfield AO, Wolfe JH. 11-year experience with anatomical and extra-anatomical repair of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg 2004;27:585-9. [Crossref] [PubMed]
- Sorelius K, Mani K, Bjorck M, Sedivy P, Wahlgren CM, Taylor P, Clough RE, Lyons O, Thompson M, Brownrigg J, Ivancev K, Davis M, Jenkins MP, Jaffer U, Bown M, Rancic Z, Mayer D, Brunkwall J, Gawenda M, Kolbel T, Jean-Baptiste E, Moll F, Berger P, Liapos CD, Moulakakis KG, Langenskiold M, Roos H, Larzon T, Pirouzram A. Wanhainen A for the European MAA collaborators. Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation 2014;130:2136-42. [Crossref] [PubMed]
- Huang YK, Chen CL, Lu MS, Tsai FC, Lin PL, Wu CH, Chiu CH. Clinical, microbiologic, and outcome analysis of mycotic aortic aneurysm: the role of endovascular repair. Surg Infect (Larchmt) 2014;15:290-8. [Crossref] [PubMed]
- Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg 2007;46:906-12. [Crossref] [PubMed]
- Chiu CH, Su LH, Chu C. Salmonella enterica serotype Choleraesuis: Epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev 2004;17:311-22. [Crossref] [PubMed]
- Guiney DG. The role of host cell death in Salmonella infections. Curr Top Microbiol Immunol 2005;289:131-50. [Crossref] [PubMed]
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709-21. [Crossref] [PubMed]
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012;12:881-7. [Crossref] [PubMed]
- Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 2013;4:107-18. [Crossref] [PubMed]
- Long R, Guzman R, Greenberg H, Safneck J, Hershfield E. Tuberculous mycotic aneurysm of the aorta: review of published medical and surgical experience. Chest 1999;115:522-31. [Crossref] [PubMed]
- Shigemitsu O, Hadama T, Miyamoto S, Anai H, Sako H. Tuberculous pseudoaneurysm of the ascending aorta with intracranial tuberculoma. J Cardiovasc Surg (Torino) 2002;43:59-62. [PubMed]
- Ting AC, Cheng SW. Femoral pseudoaneurysms in drug addicts. World J Surg 1997;21:783-6. [Crossref] [PubMed]
- Lee WK, Mossop PJ, Little AF, Fitt GJ, Vrazas JI, Hoang JK, Hennessy OF. Infected (mycotic) aneurysms: Spectrum of imaging appearances and management. Radiographics 2008;28:1853-68. [Crossref] [PubMed]
- Ramachandran Nair H, Goura P, Pitchai S, Madathipat U. Brucella-induced ruptured infrarenal dissecting abdominal aortic aneurysm. Aorta (Stamford) 2019;7:56-8. [Crossref] [PubMed]
- Huang JJ, Ruaan MK, Lan RR, Wang MC. Acute pyogenic iliopsoas abscess in Taiwan: clinical features, diagnosis, treatments and outcome. J Inf Secur 2000;40:248-55. [Crossref] [PubMed]
- Hsieh MS, Huang SC, Loh EW, Tsai CA, Hung YY, Tsan YT, Huang JA, Wang LM, Hu WY. Features and treatment modality of iliopsoas abscess and its outcome: a 6-year hospital-based study. BMC Infect Dis 2013;13:578. [Crossref] [PubMed]
- Lai YC, Lin PC, Wang WS, Lai JI. An update on psoas muscle abscess: an 8- year experience and review of literature. Int J Gerontol 2011;5:75-9. [Crossref]
- Hu SY, Hsieh MS, Chang YT, Huang CC, Tsai CA, Tsai CL, Hsu CY, Shen CH, Chang YZ. Clinical features, management, and outcome of iliopsoas abscess associated with cardiovascular disorders: a hospital- based observational case series study. BMC Musculoskelet Disord 2019;20:474. [Crossref] [PubMed]
- Nakamura T, Morimoto T, Katsube K, Yamamori Y, Mashino J, Kikuchi K. Clinical characteristics of pyogenic spondylitis and psoas abscess at a tertiary care hospital: a retrospective cohort study. J Orthop Surg Res 2018;13:302. [Crossref] [PubMed]
- Saers SJ, Scheltinga MR. Primary aortoenteric fistulae. Br J Surg 2005;92:143-52. [Crossref] [PubMed]
- Bergqvist D, Björck M. Secondary arterioenterc fistulation-a systematic literature analysis. Eur J Endovasc Surg 2009;37:31-42. [Crossref] [PubMed]
- Karhof S, van Roeden SE, Oosterheert JJ, Bleeker-Rovers CP, Renders NHM, de Borst GJ, Kampschreur LM, Hoepelman AIM, Koning OHJ, Wever PC. Primary and secondary arterial fistulas during chronic Q fever. J Vasc Surg 2018;68:1906-13. [Crossref] [PubMed]
- Lyons OT, Baguneid M, Barwick TD, Bell RE, Foster N, Homer-Vanniasinkam S, Hopkins S, Hussain A, Katsanos K, Modarai B, Sandoe JA, Thomas S, Price NM. Diagnosis of Aortic Graft Infection: A Case Definition by the Management of Aortic Graft Infection Collaboration (MAGIC). Eur J Vasc Endovasc Surg 2016;52:758-63. [Crossref] [PubMed]


