
The effect of stent compression on in-stent restenosis and clinical outcomes in iliac vein compression syndrome
Introduction
Iliac vein compression syndrome (IVCS, also known as May-Thurner syndrome or Cockett syndrome) is an anatomical variant that results in the left common iliac vein's compression by the right iliac artery. It occurs in approximately 25% of the population and is thought to cause chronic venous disease (CVD) (1-3). The symptoms of chronic venous insufficiency significantly decrease patients’ quality of life.
With the popularization of intracavitary interventional techniques and the innovation of interventional devices, endovascular treatment is currently recognized as a preferred method for treating IVCS. An increasing number of researchers have shown the safety, effectiveness, and durability of intravenous stent placement (4-9). Inevitably, stent dysfunctions, such as stent compression and in-stent restenosis (ISR), are common complications (6). Stent patency is highly affected by significant stent compression (SSC) (10). Like that of a stent in the peripheral artery (11), the development of ISR is also a factor influencing venous stent patency. It has been reported that approximately 20% of stents require reintervention due to ISR (12).
Both stent compression and ISR affect a stent’s patency; however, the effect of stent compression on ISR is unclear. This study sought to further explore the effect of stent compression on ISR and clinical outcomes by analyzing patients’ postoperative follow-up data with iliac vein stenting at our center.
Methods
Patients
This prospective study was conducted at our center with board approval. To meet the study’s inclusion criteria, patients had to have been diagnosed with IVCS associated with venous insufficiency [Clinical-Etiological-Anatomical-Pathophysiological (CEAP) ≥ C3] and aged between 18–80 years old. Patients were excluded from the study if they had right-sided iliac vein compression, thrombophilia, or a serious underlying disease. The preoperative venous clinical severity scores (VCSSs), CEAPC4–C6, and chronic venous insufficiency questionnaire (CIVIQ) scores of the patients were also collected. The ethical committee approved this study of The First Affiliated Hospital of Chongqing Medical University.
Procedure
Intravenous ultrasounds (IVUS) have not yet been popularized in China, and our center is not yet equipped with this technology. Consequently, venography was the method used to diagnose IVCS. Patients were placed under local anesthesia, and the left femoral vein was punctured, and the vascular sheath was inserted. Venographies were performed of patients’ in the anteroposterior and lateral positions (and the oblique position if necessary) to identify the left iliac vein stenosis. For patients whose percentage of stenosis could not be determined by antegrade venography, a three-dimensional digital subtraction angiography (3D-DSA) was also performed. After processing and reconstruction, the three-dimensional vascular images of the left iliac vein and inferior vena cava were obtained, and the iliac vein stenosis was observed from multiple angles.
Patients were diagnosed with IVCS if their radiography results showed the following three major signs: (I) an iliac vein filling defect or separation, stenosis >50% or occlusion; (II) the diameter of the compressed segment of the left iliac vein was significantly widened, and the display of the contrasting agent at the compressed segment became lighter; and (III) the formation of collateral circulation. There were two indications of stenting: (I) evidence of IVCS; and (II) chronic venous insufficiency (CEAP ≥ C3). The stenosis rate was calculated by measuring the compressed iliac vein’s minimum diameter and the diameter of adjacent normal vessels. The length of the iliac vein lesion was also measured. The stent was implanted when stenosis was >50%. The standards for stent implantation included that: (I) the stents were oversized (by 10–20%) to ensure proper expansion; (II) the stents exceeded the lesion (as seen on a phlebography) by at least 5 mm in both directions. A balloon catheter (12 to 14 mm, Boston Scientific Corporation, USA) was used to pre-dilate the lesion, and the diameter of the balloon was selected according to the diameter of the normal iliac vein segment (the pressure must be sufficient to dilate the lesions fully). A stent (Smart Control, Cordis, USA) was implanted with a 12 to 14 mm diameter and a length of 60 to 80 mm. Venographies were reperformed to determine the position of the stent and blood flow. If the residual stenosis in the stent was >30%, a 1:1 balloon was used for dilation. As the stent (Smart Control, Cordis, USA) had insufficient support force due to its open-cell design, the occurrence of stent compression could not be avoided even if there were full pre-dilatation and post-dilatation because of the high pressure of the compressed iliac vein. Thus, as per the standard and based on the venographies, arterial stent implantation with residual stenosis of less than 30% was deemed a technical success. Additionally, all patients underwent minimally invasive treatment of varicose veins, such as foam sclerotherapy, 1 month after stenting.
Postoperative management
Rivaroxaban (20 mg, qd) was taken by all patients for 3 months after stenting, after which patients took Rivaroxaban (10 mg, qd) for 1 year after surgery.
Follow-up
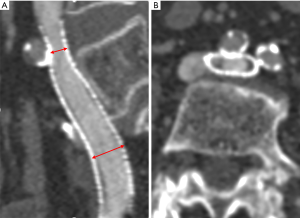
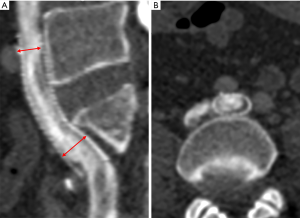
To ensure that each patient was followed-up with, a specially assigned doctor was responsible for contacting each patient. All patients were followed-up with 1, 3, and 6 months after surgery and underwent Doppler ultrasound (DU) to evaluate the occurrence of stent compression and ISR. One year after stenting, the postoperative VCSS and CIVIQ scores of all the patients were recorded and compared between the two groups to evaluate the clinical improvement of venous insufficiency. Computed tomography venography (CTV) was also performed within 1 week before or after the time point of the 1-year post-stenting to evaluate the occurrence of stent compression and ISR, including the location and degree of stent compression (i.e., the narrowest stent diameter/the widest stent diameter ×100%). In-stent stenosis of more than 50% and a lack of clinical improvement were considered an indication for reintervention (13). According to the degree of stent compression, the patients were allocated to the SSC group (stent compression ≥50%) (see Figure 1) or the insignificant stent compression (ISC) group (see Figure 2) (10). ISR was defined as the presence of a focal or diffuse filling defect (neointimal hyperplasia or thrombosis) that separated the column of contrast material within the stent from the stent wall (see Figures 1B,2B). Complete stent occlusion was diagnosed when no contrast in the CTV was observed.
Statistical analysis
All the data were analyzed using SPSS (version 21.0, IBM, NY, USA). Data are expressed as the mean ± standard deviation (
) for continuous variables. The t-test was used to compare the scores (i.e., the VCSS and CIVIQ scores) between the SSC and the ISC groups before and after stenting, and the categorical data were analyzed using the χ2 test. A P value of less than 0.05 was considered statistically significant.Results
Patients
A total of 50 patients (of whom 58% were male) were enrolled in the study. Among those, the cases of C3, C4, C5, and C6 (according to CEAP classification system) were 23, 9, 5, and 3, respectively. The median age was 58 years (range: 39 to 78 years). Based on the venographies, 50 (100%) patients had severe iliac vein stenosis (>50%), and 3 (6%) of them were found to have occluded iliac veins (mean ± standard deviation, 79.42%±9.52%; range, 64–100%). An occlusion was defined as a short segment of the left common iliac vein caused by the right common iliac artery’s compression confirmed by a 3D-DSA. These three patients had a negative D-dimer and no obvious history of deep vein thrombosis (DVT).
Fifty patients underwent stent implantation with a laser-cut nitinol stent (Smart Control, Cordis, USA). All patients completed the protocol, and the technical success rate was 100%. One month after stenting, SSC was observed in 13 patients, and ISC was observed in 27 patients. Among those patients, 4 progressed to SSC 3 months after stenting. Finally, CTVs revealed 17 patients with SSC and 33 with ISC, including 10 without stent compression at one year after surgery. Patients with persistent, recurrent, or worsening symptoms rarely accepted reintervention for economic or cultural reasons. Most of the patients in the SSC group exhibited residual symptoms of lower extremities or slow recovery of symptoms after stentings, such as recurring edemas and slow healing of ulcers. A total of 90% (36/40) of patients had compression of the stent’s proximal end due to compression of the right common iliac artery and/or posterior vertebrae, and the other 4 patients had multiple sites of compression. There was no evidence of stent migration or fracture. CTVs revealed no obvious stenosis of the external iliac vein or femoral vein in any patient.
Preoperative comparison
Table 1 presents a preoperative comparison of the baseline characteristics between the two groups. No patient was found to have thrombophilia, and there were no statistically significant differences in age or sex distribution between the SSC and ISC groups, nor were there statistically significant differences in VCSS, CEAPC4–C6, or CIVIQ scores between the two groups.

Full table
Postoperative comparison
As Table 2 shows, there was a significant difference in the incidence of ISR between the SSC group (52.9%) and the ISC group (21.2%) (P=0.023). One month after stenting, mild ISR was detected in 6 and 3 patients in the SSC and ISC groups, respectively; another 3 and 4 patients progressed to mild ISR 3 months after stenting in the two groups. As there was no evidence of ISR progression during the follow-up period, no reintervention was required. No significant difference was found in the occurrence of stent occlusion between the two groups (P=0.999). In both groups, 1 patient developed stent occlusion 1 year after surgery. That patient in the ISC group showed no obvious ISR, and the level of stent compression remained at 30% according to postoperative DU follow-up. The probable reason for stent occlusion was the patient’s self-discontinuation of the anticoagulant drugs 6 months after surgery.

Full table
Conversely, the 1 patient in the SSC group sustained stent compression of 65% and unprogressed mild ISR at the first half-year follow-up. The exact cause and time of the stent occlusion were unknown. There were also significant differences between the two groups in terms of VCSSs (P=0.04) and CIVIQ scores (P=0.001). All of the patients had consistent standards for stent implantation, and the diameter, length, and number of stents had no effects on the degree of stent compression (see Table 3).

Full table
Discussion
In recent years, iliac vein stenting has become the preferred method for treating IVCS in China. Stent compression and ISR are common postoperative complications. In the present study, 80% (40/50) of the patients had varying degrees of stent compression, and SSC was highly associated with ISR incidence.
Due to the lack of stents designed specifically for venous obstruction at present in the Chinese market, an arterial-designed stent is usually used to replace venous stents (14-17). However, there are obvious differences in the anatomy and hemodynamics in physiological and pathophysiological conditions between the arterial and venous systems (14). As a result, veins are more prone to external forces due to their less supported walls. The stent release principle was that the length of the proximal stent into the inferior vena cava should be as short as possible to reduce the interference to the contralateral blood flow (18). In our study, almost all of the stents extended several millimeters into the inferior vena cava. Despite this, the stent segment at the compression point was still unable to resist external pressure. Raju et al. (19) and Cho et al. (10) reported that the proportion of limbs showing stent compression was 25% and 33%, respectively. The arterial-designed stent with a relatively weak outward radial force is insufficient to resist the iliac vein’s external compression and may not be suitable for the venous system. Some dedicated venous stents exist in the Western market at present, such as Sinus Obliquus (Optimed, Ettlingen, Germany), which were designed with a closed-cell at the proximal end to enhance the support force and reduce the occurrence of stent compression. However, even in a dedicated venous stent, an approximately oval configuration is found at high compression levels in most cases (14).
Stent compression is often associated with ISR in iliac venous stents. Raju et al. showed that in all 103 limbs with ISR, 25% showed stent compression (19). Another study revealed that ISR and stent compressions occurred in 107 of 177 (60%) of patients who had undergone reintervention procedures (12). A smaller cross-sectional area (resulting from stent compression) is more likely to cause ISR (20) after stenting. Cho et al. found that SSC was the only factor associated with the development of stent occlusion (10). Further, Barbati et al. demonstrated that the reduction of stent lumen over time has a strong effect on stent occlusion (13). The present study outcomes showed that the occurrence of ISR in the SSC group (52.9%) was significantly higher than that in the ISC group (21.2%), which indicates that the significant compression of the stent is more likely to cause ISR.
A previous study showed that the possible risk factors for the development of ISR appear to be thrombophilia, prior thrombosis, and the use of a long stent in chronic iliac vein stenosis or occlusion cases (21). In our study, all of the patients who participated had non-thrombotic iliac vein lesions (NIVL) without thrombophilia, and there was no statistically significant difference in the use of stents between the two groups. Thus, stent compression appears to be a risk factor of ISR. Stent compression leads to a reduced lumen area and changes in hemodynamics (e.g., flow, flow rate, and pressure), which are the possible causes of ISR and even stent occlusion. The specific mechanism of stent compression leads to ISR or even stent occlusion still needs to be further explored.
How ISR progresses and its relationship with stent occlusion remain unclear. Jayaraj et al. analyzed potential risk factors for stent occlusion and found that ISR was not a statistically significant predictor. Additionally, they stated that ISR showed few signs of progress during the follow-up years, and the progression of ISR to stent occlusion was infrequent (22). Neglén and Raju found the significant progression of ISR in individual cases, and patients with stent occlusion had similar risk factors for the development of severe ISR, but the cause-effect relationship between the development of ISR and stent occlusion was unknown (20). Thus, it is possible that stent occlusion is not a result of ISR’s continuous development over time. In the present study, we found that SSC was associated with ISR's occurrence rate; however, we were unable to observe the progression of ISR to stent occlusion. This may be because (I) all of the patients recruited had NIVL, which has a lower occlusion rate than thrombotic cases (23,24); (II) all of the patients were prescribed postprocedural anticoagulation medication; and (III) our study had a short follow-up period.
SSC may cause venous hypertension and residual symptoms of the lower extremities (12). In the present study, we observed that the differences between the SSC and ISC groups in terms of VCSSs and CIVIQ scores were significant, indicating that SSC affects the outcomes of clinical symptoms of lower limb venous insufficiency. Thus, reducing the incidence of stent compression is of great importance for the relief of clinical symptoms after iliac vein stenting.
Our study was limited by its short follow-up period and the comparatively small sample size. The mid- and long-term effects of stent compression on ISR and clinical outcomes and ISR progression should be subject to further research. Additionally, due to the lumen’s elliptical shape after stenting and the irregular shape of ISR in the stent, we were unable to assess the specific extent of ISR and the overall degree of stenosis when stent compression presents with ISR. IVUS showed more advantages in diagnosing IVCS, and it is the gold standard for diagnosing iliac vein lesions (12,19,25). Finally, we could only use venographies to diagnose IVCS, which may have produced inaccurate data in the diagnoses.
Conclusions
Stent compression is a common complication after iliac vein stenting. Attempts should be made to prevent the occurrence of severe stent compression, as it has a strong effect on the incidence of ISR after iliac stenting according to the results of our study. Further, severe stent compression hinders the postoperative relief of clinical symptoms. Thus, a dedicated venous stent with sufficient radial resistive force, crush resistance, and outward radial force may be needed to treat patients with iliac vein obstruction in the future to reduce the occurrence of stent compression after iliac stenting.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-915). The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethical committee of The First Affiliated Hospital of Chongqing Medical University. Written informed consent forms were obtained from the patients for the publication of their data and any accompanying images. Copies of these written consent forms are available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mathur M, Cohen M, Bashir R. May-Thurner Syndrome. Circulation 2014;129:824-5. [Crossref] [PubMed]
- Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: A permissive role in pathogenicity. J Vasc Surg 2006;44:136-43. [Crossref] [PubMed]
- Stuck AK, Kunz S, Baumgartner I, Kucher N. Patency and Clinical Outcomes of a Dedicated, Self-Expanding, Hybrid Oblique Stent Used in the Treatment of Common Iliac Vein Compression. J Endovasc Ther 2017;24:159-66. [Crossref] [PubMed]
- Rossi FH, Kambara AM, Izukawa NM, Rodrigues TO, Rossi CB, Sousa AG, Metzger PB, Thorpe PE. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord 2018;6:183-91. [Crossref] [PubMed]
- Abou Ali AN, Avgerinos ED, Chaer RA. Role of Venous Stenting for Iliofemoral and Vena Cava Venous Obstruction. Surg Clin North Am 2018;98:361-71. [Crossref] [PubMed]
- van Vuuren TMAJ, Doganci S, Wittens CHA. Patency rates and clinical outcomes in a cohort of 200 patients treated with a dedicated venous stent. J Vasc Surg Venous Lymphat Disord 2018;6:321-9. [Crossref] [PubMed]
- Juhan C, Hartung O, Alimi Y, Barthélemy P, Valerio N, Portier F. Treatment of nonmalignant obstructive iliocaval lesions by stent placement: mid-term results. Ann Vasc Surg 2001;15:227-32. [Crossref] [PubMed]
- Raju S, Owen S, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg 2002;35:8-15. [Crossref] [PubMed]
- Mahnken AH, Thomson K, de Haan M, O'Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol 2014;37:889-97. [Crossref] [PubMed]
- Cho H, Kim JW, Hong YS, Lim SH, Won JH. Stent Compression in Iliac Vein Compression Syndrome Associated with Acute Ilio-Femoral Deep Vein Thrombosis. Korean J Radiol 2015;16:723-8. [Crossref] [PubMed]
- Loffroy R, Edriss N, Goyault G, Chabanier A, Pernes JM, Sauguet A, Touil M, Woerly B, Pongas D, Chevallier O, Falvo N, Galland C, Midulla M, Garnier N, Guenfoudi MP, Boulin M, Aho-Gléglé S, Bost S. Percutaneous mechanical atherothrombectomy using the Rotarex®S device in peripheral artery in-stent restenosis or occlusion: a French retrospective multicenter study on 128 patients. Quant Imaging Med Surg 2020;10:283-93. [Crossref] [PubMed]
- Raju S, Tackett P Jr, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg 2009;49:511-8. [Crossref] [PubMed]
- Barbati ME, Gombert A, Toonder I, van Vuuren TM, Schleimer K, Grommes J, Wittens CH, Jalaie H. Detecting stent geometry changes after venous recanalization using duplex ultrasound. Phlebology 2019;34:8-16. [Crossref] [PubMed]
- de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Short-Term Clinical Experience with a Dedicated Venous Nitinol Stent: Initial Results with the Sinus-Venous Stent. Eur J Vasc Endovasc Surg 2015;50:518-26. [Crossref] [PubMed]
- Xue GH, Huang XZ, Ye M, Liang W, Zhang H, Zhang JW, Zhang BG. Catheter-directed thrombolysis and stenting in the treatment of iliac vein compression syndrome with acute iliofemoral deep vein thrombosis: outcome and follow-up. Ann Vasc Surg 2014;28:957-63. [Crossref] [PubMed]
- Wen-da W, Yu Z, Yue-Xin C. Stenting for chronic obstructive venous disease: A current comprehensive meta-analysis and systematic review. Phlebology 2016;31:376-89. [Crossref] [PubMed]
- Shi WY, Gu JP, Liu CJ, He X, Lou WS. Endovascular treatment for iliac vein compression syndrome with or without lower extremity deep vein thrombosis: A retrospective study on mid-term in-stent patency from a single center. Eur J Radiol 2016;85:7-14. [Crossref] [PubMed]
- Caliste XA, Clark AL, Doyle AJ, Cullen JP, Gillespie DL. The incidence of contralateral iliac venous thrombosis after stenting across the iliocaval confluence in patients with acute or chronic venous outflow obstruction. J Vasc Surg Venous Lymphat Disord 2014;2:253-9. [Crossref] [PubMed]
- Raju S, Davis M. Anomalous features of iliac vein stenosis that affect diagnosis and treatment. J Vasc Surg Venous Lymphat Disord 2014;2:260-7. [Crossref] [PubMed]
- Neglén P, Raju S. In-stent recurrent stenosis in stents placed in the lower extremity venous outflow tract. J Vasc Surg 2004;39:181-7. [Crossref] [PubMed]
- Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg 2013;57:1163-9. [Crossref] [PubMed]
- Jayaraj A, Crim W, Knight A, Raju S. Characteristics and outcomes of stent occlusion after iliocaval stenting. J Vasc Surg Venous Lymphat Disord 2019;7:56-64. [Crossref] [PubMed]
- Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979-90. [Crossref] [PubMed]
- Razavi MK, Jaff MR, Miller LE. Safety and Effectiveness of Stent Placement for Iliofemoral Venous Outflow Obstruction: Systematic Review and Meta-Analysis. Circ Cardiovasc Interv 2015;8:e002772 [Crossref] [PubMed]
- Gagne PJ, Gasparis A, Black S, Thorpe P, Passman M, Vedantham S, Marston W, Iafrati M. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord 2018;6:48-56.e1. [Crossref] [PubMed]


