Fahr disease: use of susceptibility-weighted imaging for diagnostic dilemma with magnetic resonance imaging
Introduction
Fahr disease (FD), idiopathic bilateral striopallidodentate calcinosis, is a rare neurodegenerative disease that is characterized by symmetric extensive intracranial calcifications, predominantly in the basal ganglia (1). Magnetic resonance imaging (MRI) is exquisitely sensitive in detecting brain abnormalities and thus the best diagnostic method in the central nervous system. However, it is difficult to reliably identify calcifications by routine MRI because calcifications appear with various signal intensities on conventional MRI sequences (2,3). Therefore, computed tomography (CT), in general, is the preferable modality for detecting and localizing the extent of intracranial calcifications. Also, in cases of FD with the presence of calcifications that are suspicious on MRI, CT is considered to be critical for accurate diagnosis (2).
Susceptibility-weighted imaging (SWI) is a fully velocity-compensated 3D gradient-echo (GE) MR sequence with special phase and magnitude processing (4,5). SWI has a high sensitivity for blood products, nonheme iron, and calcifications within the brain and thus provides additional diagnostic information in various neurologic disorders, including stroke, trauma, occult vascular malformations, vasculopathies, neoplasms, coagulopathic or other hemorrhagic disorders, and neurodegenerative disorders. Furthermore, SWI phase images have the ability to recognize definitively the existence of calcium components, with higher sensitivity compared to other MRI sequences.
In this article, we present two cases of FD with different manifestations and neuroimaging in different age groups and genders, which were diagnosed by SWI and confirmed with CT, and we discuss the contribution of SWI in the diagnosis of FD.
Case report
Case 1
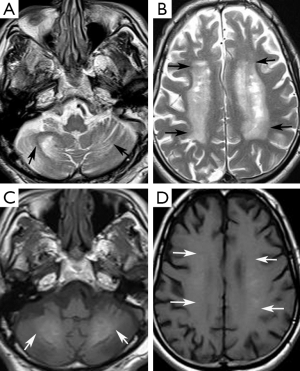
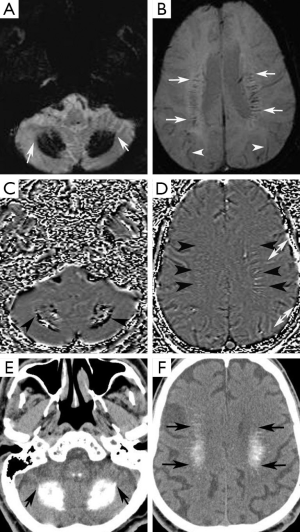
A 74-year-old man presented at our neurology outpatient clinic with headache, progressive short-term memory deficit for 1 year, and weakness of the upper and lower extremities with tremor. Neurological examination with standard neuropsychological tests showed slight cognitive deficit, characterized by memory impairment. Cranial MRI revealed diffuse symmetrical hyperintense signal in the bilateral basal ganglia, dentate nuclei, and also involving the periventricular white matter and centrum semiovale bilaterally on T2-weighted and FLAIR images (Figure 1A,B). T1-weighted images showed mild hyperintense signal changes in the dentate nuclei and minimal hyperintensity in the centrum semiovale (Figure 1C,D). In addition, cerebral and cerebellar atrophy was noted (Figure 1). These signal changes were hypointense on SWI minimal intensity projection (minIP) and phase images, consistent with calcification (Figure 2A-D). Brain CT imaging confirmed calcifications. (Figure 2E,F). Electroencephalogram (EEG) was normal. Laboratory data including metabolic tests which were performed to investigate calcium deposition disorders (including Ca/P, parathormone hormone) were unremarkable. On the basis of laboratory test results, neuroimaging findings, and clinical data, FD was diagnosed.
Case 2
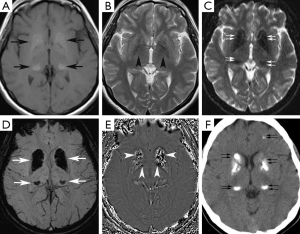
A 37-year-old woman presented at our neurology outpatient clinic with headache, vertigo, ataxia, tremor, and confusion without loss of consciousness 2 days ago. Neurological examination showed postural kinetic tremor. Cranial MRI revealed symmetrical hyperintense signal mostly in the basal ganglia and bilateral thalami, also involving less prominently periventricular white matter and bilateral dentate nuclei on T1-weighted images (Figure 3A). T2-weighted images showed mild hypointense signal changes in the basal ganglia (Figure 3B). These signal changes were hypointense on GE images (Figure 3C), SWI minIP (Figure 3D) and phase images (Figure 3E), consistent with calcification. Brain CT imaging confirmed calcifications (Figure 3F). EEG was normal. Calcium metabolism abnormalities were excluded by laboratory tests. The final diagnosis was FD.
Discussion
FD is a well-defined, rare clinical entity with sporadic or familial manifestation (1,6). As intracranial calcifications tend to favor the basal ganglia and dentate nuclei, a definitive term, “bilateral striopallidodentate calcinosis”, has been proposed to be most appropriate (1).
The combined data provided from 99 patients with proven calcium deposits revealed that approximately two-thirds of patients with FD were symptomatic (7). The male:female ratio was 2:1. The most common manifestation was movement disorders (55%), particularly Parkinsonism, accounting for over half of movement disorders, while hyperkinetic movement disorders comprised the rest. Cognitive impairment was the second most common presentation, followed by cerebellar impairment and speech disorder. Other nonspecific overlapping neurologic symptoms included pyramidal signs, psychiatric features, gait disorders, seizures, neuropsychological impairment and behavioral abnormalities, stroke-like events, and headache. Typical clinical presentation has been reported to begin in the 4th to 5th decades; however, patients may present with diverse manifestations at different ages, as seen in our patients (6).
FD is characterized by extensive calcifications, which are typically bilateral and symmetrical and are frequently located in the basal ganglia, dentate nuclei, thalamus, brain stem, centrum semiovale, and subcortical white matter (1,8). The pathogenesis of FD is unclear. Earlier studies suggested that T2 hyperintense areas may reflect a slowly progressive inflammatory or metabolic brain process, which subsequently calcifies, because MRI findings have been better correlated with neurologic deficits than calcification on CT (2). However, in our cases, T2 hyperintensities were consistent with calcifications on SWI phase images, which might not be diagnosed by conventional MRI. The neurological symptoms may be better related to the extent of calcifications observed on SWI phase images. The predilection of calcium deposits to the basal ganglia remains unknown, but the basal ganglia is a well-known target for many other deposits and various minerals (bilirubin in kernicterus, copper in Wilson’s disease, and manganese in Parkinsonism, etc.) (1).
In FD, calcified components are always hyperdense on CT (8). However, the MR signal of these calcifications may vary on conventional sequences due to the difference in the composition of calcium deposits, which may include other minerals, such as zinc, magnesium, and iron, and mucopolysaccharides and proteins binding the mineral ions, in addition to calcium (7,8). Therefore, calcification cannot be reliably identified on MRI, and CT is considered more sensitive than MRI for recognition of the calcium deposits in FD.
The calcium depositions in FD can be best detected as decreased signal intensity on GE images or SWI sequence on MRI (9). However, both calcifications and hemorrhages appear as hypointense spots and cannot be differentiated by GE images (3). On the other hand, SWI phased images have been reported to reliably differentiate calcification from hemorrhage (3,4,10). Calcifications tend to be diamagnetic and blood products paramagnetic in comparison to brain parenchyma, and thus they appear with the opposite signal intensity on SWI phase images. A practical solution for inexperienced users might be to use veins as a reference on SWI phase images such that the signal caused by calcium is opposite to that of the paramagnetic veins.
SWI has been found to be more accurate in identifying and quantifying the calcifications than conventional MRI sequences (3,10). Therefore, SWI phase images can provide recognition of intracranial calcifications comparable to CT. However, in the literature, patients were diagnosed as having FD on the basis of the presence of extensive intracranial calcifications on CT (7,8). Cranial CT has been suggested to detect calcium deposits in the presence of clinical symptoms suggestive of FD, such as movement disorder, cognitive impairment, and ataxia (1). To our knowledge, only one review has defined SWI in MRI diagnosis of FD (9). Kozic et al. reported three patients with intracranial calcifications (pseudohypoparathyroidism, FD, hypercalcemia) and concluded that MR including GE sequence, without CT, can be a confusing and even misleading diagnostic method for the detection of both extensive and subtle cerebral calcifications (11). However, SWI phase images can be helpful to identify intracranial calcifications as well as CT, as shown in these cases, and may prevent the requirement for CT to confirm calcifications.
Possible artifacts that may accompany SWI phase images should be considered in evaluating the data for clinical applications (3,10). Aliasing is the major disadvantage, which can be reduced by imaging with a shorter echo and/or double echo time. The second disadvantage, dipole field associated with a sphere, is the appearance of both a negative and positive phase surrounding a larger diamagnetic object, which can be removed by suppressing local magnetic fields or susceptibilities by reprocessing images. In addition, a small calcification with high concentration may create more chemical shift, leading to usual positive phase shift but a stronger field effect that results in aliasing in the center of the lesion. This can be solved by more appropriate phase conversion to local susceptibility.
The diagnosis of FD is provided by the presence of extensive bilateral symmetric intracranial calcifications and ruling out calcium metabolism abnormalities and developmental defects (1). The major differential diagnosis includes hypoparathyroidism. Secondary extensive bilateral cerebral calcifications have also been described in a variety of developmental, genetic, infectious metabolic, and other conditions.
In conclusion, CT is the modality of choice in the diagnosis of FD. However, MRI, which is the preferred diagnostic technique for neuroimaging, may be confusing for the detection of calcifications. SWI phase images can detect calcifications and provide the diagnosis of FD. Therefore, we suggest integrating SWI with the MRI protocol to identify calcifications in suspicion of neurodegenerative disorders.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Manyam BV. What is and what is not ‘Fahr’s disease’. Parkinsonism Relat Disord 2005;11:73-80. [PubMed]
- Avrahami E, Cohn DF, Feibel M, Tadmor R. MRI demonstration and CT correlation of the brain in patients with idiopathic intracerebral calcification. J Neurol 1994;241:381-4. [PubMed]
- Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with MRI using susceptibility-weighted imaging: a case study. J Magn Reson Imaging 2009;29:177-82. [PubMed]
- Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 2009;30:232-52. [PubMed]
- Şahin N, Solak A, Genç B, Bilgiç N., Susceptibility-Weighted MR. Imaging: Added value of susceptibility signals in diagnosis of hemorrhagic lesions of the brain. Turkish Journal of Cerebrovascular Diseases 2014;20:77-86.
- Ashtari F, Fatehi F. Fahr’s disease: variable presentations in a family. Neurol Sci 2010;31:665-7. [PubMed]
- Manyam BV, Walters AS, Narla KR. Bilateral striopallidodentate calcinosis: clinical characteristics of patients seen in a registry. Mov Disord 2001;16:258-64. [PubMed]
- Faria AV, Pereira IC, Nanni L. Computerized tomography findings in Fahr’s syndrome. Arq Neuropsiquiatr 2004;62:789-92. [PubMed]
- Bekiesinska-Figatowska M, Mierzewska H, Jurkiewicz E. Basal ganglia lesions in children and adults. Eur J Radiol 2013;82:837-49. [PubMed]
- Berberat J, Grobholz R, Boxheimer L, Rogers S, Remonda L, Roelcke U. Differentiation between calcification and hemorrhage in brain tumors using susceptibility-weighted imaging: a pilot study. AJR Am J Roentgenol 2014;202:847-50. [PubMed]
- Kozic D, Todorovic-Djilas L, Semnic R, Miucin-Vukadinovic I, Lucic M. MR imaging - an unreliable and potentially misleading diagnostic modality in patients with intracerebral calcium depositions. Case report. Neuro Endocrinol Lett 2009;30:553-7. [PubMed]


