
Magnetic resonance imaging-guided microwave ablation for lung tumor: a case report
Introduction
Lung cancer has taken the place of liver cancer as the most common malignancy in China, and is the leading cause of cancer-related mortality among the Chinese population (1). Thoracoscopic surgery is the traditional treatment for localized non-small cell lung cancer (NSCLC). However, 25% of patients diagnosed with lung cancer are deemed to be inoperable due to poor cardiopulmonary reserve and the presence of medical comorbidities (2). Radiofrequency (RF) energy has been used successfully for local control and palliative treatment of various soft tissue malignancies, including thoracic neoplasms. Over the past decade, other image-guided, minimally invasive, and lung-sparing therapies, such as stereotactic body radiation therapy (SBRT), radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, chemical ablation, and laser ablation, have also been widely applied in patients with inoperable lung cancer (3-6).
MWA is a well-tolerated technique that involves an acceptable level of surgical trauma. It has all the benefits of RF ablation and also offers substantial advantages (7). MWA is widely used in the treatment of many malignant carcinomas including, among others, liver cancer, lung cancer, and metastatic bone tumors (7). Patient groups treated with MWA for stage I NSCLC have shown similar outcomes to those treated with RFA, indicating that MWA may be an effective minimally invasive treatment for lung cancers (8,9). In most cases, ablation is performed with computed tomography (CT) guidance, while magnetic resonance (MR)-guided ablation is rare. At present, there are no reports of MR-guided MWA being applied for lung cancer. Therefore, the purpose of our study was to retrospectively evaluate the safety, practicality, and efficacy of MR imaging (MRI)-guided MWA in the case of a patient with intraparenchymal pulmonary malignancies.
Case presentation
On December 1, 2017, a 69-year-old male was admitted to the Department of Thoracic Surgery of the Affiliated Cancer Hospital of Nanjing Medical University. The patient had no smoking history or clinical symptoms. He had previously been diagnosed with lung adenocarcinoma in the right lower lobe in January, 2016 (Figure 1). A CT-based radiomics analysis for this patient indicated clinical stage IIIA lung cancer, and spirometry revealed a forced expiratory volume in 1 second (FEV1) of 1.6 L (52% predicted) and a forced vital capacity (FVC) of 3.0 L (75% predicted).
The patient refused to undergo radical surgical intervention. Subsequently, he was administered pemetrexed (PEM) 500 mg/m2, day 1, and cisplatin (DDP) 20.0 mg/m2, day 1–day 3, every 21 days for a total of six cycles of chemotherapy, starting in February, 2016. A follow-up examination in April 2017 indicated an enlarged lesion in the right lower lung. The patient underwent two CT-guided MWA sessions; however, the tumor continued to grow.
On December 4, 2017, the patient received percutaneous MWA under the guidance of MRI at our hospital. Our surgical team comprised two interventional radiologists with more than 8 years of experience in thoracic surgery and proficiency in managing pneumothorax, hemoptysis, and other surgical complications, as well as a technician and an experienced radiologist. All preoperative and postoperative MRI scans were performed using a 3.0 T MR scanner (PHILIPS-Ingenia 3.0T, Amsterdam, The Netherlands).
Before his procedure, the patient was instructed to fast for 6 hours and underwent complete routine lab tests. MRI-guided MWA was performed using an ECO-100A MWA system (ECO Medical Instrument Co., Ltd. Nanjing, China; CFDA Certificated No.: 20173251268) and a 16-gauge cooled-shaft ceramic antenna. All metal equipment was kept in a safe area by the radiologist. The power output was set at 50–60 W for the entire operation. The procedure was performed in the interventional radiology room. Based on the location of the lesion, the patient was placed in the supine position on the surgical bed. A skin marker was applied to locate the needle insertion site. In order to obtain a satisfactory image, the patient was instructed to hold his breath for approximately 10 seconds during the scan so the location of the puncture could be determined. T2-weighted images were used to confirm the needle placement (Figure 2A). The distance from the skin marker to the center of the tumor location was 91 mm in the axial, coronal, and sagittal images. These measurements were helpful in determining the relative spatial location of the needle tip and the irregular tumor. Satisfactory local anesthesia was achieved with 1% lidocaine. According to the preoperative plan, the 16-gauge microwave antenna was placed into the tumor at an angle of approximately 90˚ in a step-by-step manner. To ensure that the ablation antenna had been placed in the appropriate position, another MRI scan was performed after the needle had been inserted [T1-weighted skin echo (T1W_SE) images, Figure 2B]. Two 8-minute ablation cycles were applied to achieve an ablation zone that was 5–10 mm larger than the tumor site (Figure 2C). Subsequently, a track ablation using 20 W was performed to prevent tumor implantation metastasis and to stop bleeding at the puncture site. The patient’s vital signs including blood pressure, oxygen saturation, and heart rate were stable throughout the procedure. No pneumothorax, severe hemoptysis, or pleural effusion occurred during the operation. Following the removal of the ablation antenna, the patient was returned to the ward, and routine oxygen was given, along with other monitoring measures, under the care of a nurse.
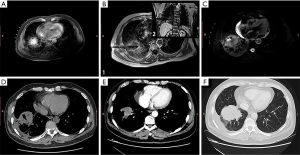
A follow-up CT scan was performed immediately after ablation to assess the efficacy of the MRI-guided MWA (Figure 2D). An ideal ablation zone, measuring 53 mm × 54 mm, was observed. CT and MRI follow-up at 6 months postoperatively revealed a significant reduction in the ablation zone. The tumor size had decreased, and measured 43 mm × 32 mm (Figure 2E). However, at the 18-month postoperative evaluation, the tumor size had increased to 43 mm × 45 mm on CT (Figure 2F). Up to this point, the patient is still alive and has reported no obvious clinical symptoms.
Discussion
Numerous studies have reported that MWA is a novel minimally invasive approach for patients with primary lung tumors and pulmonary metastasis who are not suitable for surgical resection (8-10). In recent years, CT-guided percutaneous MWA has been widely established as a complementary therapy for the treatment of thoracic malignancies. Our research adds to the existing body of knowledge and confirms that MRI-guided MWA can effectively destroy tumor cells, as suggested by other studies (11).
Compared with CT-guided MWA, MRI-guided MWA has obvious advantages. In particular, the outstanding advances made in MRI can reduce or eliminate artifacts resulting from breathing and heart function. The application of MRI is a rapidly growing field, and with the recent development of diagnostic modalities, MRI has come to play an important role in the diagnosis of thoracic disease (12,13). MRI is an established alternative to CT for the evaluation of thoracic vasculature, as well as mediastinal, hilar, and chest wall abnormalities, and can be performed without contrast agents (14). It can also avoid injury to pulmonary blood vessels and reduce the complications of hemoptysis during puncture. Images of the cross section, sagittal plane, coronal plane, and any tangent plane can be obtained directly from an MRI, which facilitates more precise guidance of the antenna to the lesion and helps to avoid multiple procedures that expose patients to potentially ionizing radiation.
CT is commonly used for evaluation following MWA therapy on the basis of morphologic criteria (15,16), as there are no quantitative identification parameters to evaluate. The obvious metal artifacts produced by the MWA antenna during CT scanning can easily cover the lesions, which is likely to lead to incomplete ablation. This may explain the failure of the previous two CT-guided ablations in the case reported here. With MRI-guided MWA, the procedure can be monitored in near real time, allowing the entire periphery of the treatment effects to be assessed during the procedure. In our patient, T2-weighted image (T2WI) sequence scanning was routinely performed during the two 8-minute ablation periods to monitor changes in the ablation zone as well as secondary changes, such as peripheral exudation. For most lesions, multiple step ablation is required. Following the ablation of each ablation site, it is impossible to perform repeated contrast-enhanced scans within a short period of time. Therefore, in the continuous ablation process, the T2WI sequence, which consumes less time and is relatively sensitive to the response of the lesion and the exudation around the lesion after ablation, was adopted to monitor the intraoperative ablation effect. The sequence scanning time, acquisition time, was 14.9 seconds, while its spatial resolution, voxel, was 1.5 mm × 1.7 mm × 3.0 mm.
Through MRI evaluation, we observed an early increase in the volume of the ablated zone (a low signal intensity on T2-weighted MR images which is surrounded by a small hyperacute signal). Also, the difference between the ablated and unablated tissue is significantly more obvious on MRI than on CT. MRI has also been demonstrated to clearly reflect changes in the ablation zone after RFA of liver lesions (17), which is consistent with our findings. MRI guidance allows physicians to determine how a lesion has changed in multiple planes in real time (18). Moreover, the potential radiation dose from multiple CT scans can be avoided.
As with all CT-guided MWA therapies, MRI guidance is associated with specific risks. Due to the low proton density of the lung and the fast signal decay at air-tissue interfaces, patients need to be able to hold their breath for a certain period of time to enhance the quality of the MRI (19). In our study, the patient was instructed to hold his breath at normal end-expiration to avoid motion-related artifacts. Considering the requirement of patient cooperation during the MRI process and the long acquisition time of the MRI system compared with CT, effective preoperative education for patients is crucial. As with CT-guided percutaneous MWA, pneumothorax, hemoptysis, and uncontrolled pain are the most common complications of MRI-guided MWA (20). In this case, the patient had hemoptysis, presumably caused by ruptured capillaries after the insertion of the probe. Fortunately, the transient symptoms disappeared within 24 hours postoperatively.
This case study has several limitations that should be noted. Firstly, the targeted lesions were not confirmed pathologically post ablation. Another limitation was the relatively insufficient continuous follow-up observations. Lastly, there were no CT follow-up images at 1 and 3 months postoperatively.
In conclusion, MRI-guided percutaneous MWA may be a suitable adjunctive therapeutic option for patients with advanced lung cancer. Comprehensive treatment plans combining MWA with other treatment strategies can achieve good therapeutic results for patients.
Acknowledgments
Funding: The study was supporting by the Health Planning Commission of Jiangsu Province (No. BE2017758) and the Scientific Research Project of Health Commission of Jiangsu Province (No. BJ18034).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-667). The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Medical Ethics Committee of Jiangsu Cancer Hospital, and informed consent was obtained from the patient. Written informed consent was also obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, Zhao R, Duan Y, Zeng Z, Li X, Li G, Xiong W, Zhou M. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci 2019;62:640-7. [Crossref] [PubMed]
- DeShazer C. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 2000;342:518-9. [PubMed]
- Okuma T, Matsuoka T, Yamamoto A, Oyama Y, Hamamoto S, Toyoshima M, Nakamura K, Miki Y. Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution. Cardiovasc Intervent Radiol 2010;33:787-93. [Crossref] [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, Wright CD, Donahue DM, Wain JC, Mathisen DJ, Shepard JA. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K, Araki T. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [Crossref] [PubMed]
- Li X, Fan W, Zhang L, Zhao M, Huang Z, Li W, Gu Y, Gao F, Huang J, Li C, Zhang F, Wu P. CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: preliminary results. Cancer 2011;117:5182-8. [Crossref] [PubMed]
- Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol 2013;57:466-74. [Crossref] [PubMed]
- Yang X, Ye X, Zheng A, Huang G, Ni X, Wang J, Han X, Li W, Wei Z. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol 2014;110:758-63. [Crossref] [PubMed]
- Wei Z, Ye X, Yang X, Zheng A, Huang G, Li W, Ni X, Wang J, Han X. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol 2015;38:135-42. [Crossref] [PubMed]
- Li J, Qu J, Zhang H, Wang Y, Zheng L, Geng X, Zhao Y, Li H. 3.0T MRI for long-term observation of lung nodules post cryoablation: a pilot study. Cancer Imaging 2017;17:29. [Crossref] [PubMed]
- Lu NH, Hung CM, Liu KY, Chen TB, Huang YH. Diagnosed chest lesion on diffusion-weighted magnetic resonance images using apparent diffusion coefficients. J Xray Sci Technol 2016;24:133-43. [Crossref] [PubMed]
- Souza CA. MRI of the chest: review of imaging strategies. Radiol Bras 2015;48:V-VI. [Crossref] [PubMed]
- Puderbach M, Hintze C, Ley S, Eichinger M, Kauczor HU, Biederer J. MR imaging of the chest: a practical approach at 1.5T. Eur J Radiol 2007;64:345-55. [Crossref] [PubMed]
- Steinke K, King J, Glenn D, Morris DL. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr 2003;27:750-7. [Crossref] [PubMed]
- Bojarski JD, Dupuy DE, Mayo-Smith WW. CT imaging findings of pulmonary neoplasms after treatment with radiofrequency ablation: results in 32 tumors. AJR Am J Roentgenol 2005;185:466-71. [Crossref] [PubMed]
- Onishi H, Matsushita M, Murakami T, Tono T, Okamoto S, Aoki Y, Iannaccone R, Hori M, Kim T, Osuga K, Tomoda K, Passariello R, Nakamura H. MR appearances of radiofrequency thermal ablation region: histopathologic correlation with dog liver models and an autopsy case. Acad Radiol 2004;11:1180-9. [Crossref] [PubMed]
- Galia M, Albano D, Narese D, Patti C, Chianca V, Di Pietto F, Mule A, Grassedonio E, La Grutta L, Lagalla R, Midiri M. Whole-body MRI in patients with lymphoma: collateral findings. Radiol Med 2016;121:793-800. [Crossref] [PubMed]
- Biederer J, Both M, Graessner J, Liess C, Jakob P, Reuter M, Heller M. Lung morphology: fast MR imaging assessment with a volumetric interpolated breath-hold technique: initial experience with patients. Radiology 2003;226:242-9. [Crossref] [PubMed]
- Padda S, Kothary N, Donington J, Cannon W, Loo BW Jr, Kee S, Wakelee H. Complications of ablative therapies in lung cancer. Clin Lung Cancer 2008;9:122-6. [Crossref] [PubMed]



