
Near-infrared temperature-switchable fluorescence nanoparticles
Introduction
Near infrared (NIR) fluorescence imaging and sensing has been attracting much attention in biomedical applications during the past decades due to its non-invasive operation, high sensitivity and specificity, non-ionizing radiation as well as deep penetration in biological tissue (1). In comparison to other imaging techniques, such as ultrasound, X-ray, magnetic resonance imaging (MRI), and positron-emission tomography (PET), fluorescence imaging provides benefits such as low cost, bio-safety, and fast detection. Also, fluorescence imaging is capable of multicolor imaging and thus it could track many interesting cellular and/or molecular-level biological interactions (2).
Recently, switchable fluorescence probes (SFPs) have gained great interest for detecting specific environmental changes in biological tissue. They have high sensitivity and specificity to a certain stimulus (3-5). A SFP usually stays at an “off” state so its fluorescence is weak. When a stimulus is applied, it can be switched “on” to emit strong fluorescence. The types of the stimulus can be physical (such as temperature), chemical (such as pH) or biological (such as biomolecule interaction) (4). Among various SFPs, one of them is the temperature-switchable fluorescence probe (T-SFP) (6-12). When a polarity- and viscosity-sensitive fluorophore is encapsulated in temperature-sensitive polymers, the fluorescence could become temperature-sensitive. This is because when the temperature rises over the lower critical solution temperature (LCST) of the polymers, the polymers experience a phase transition from hydrophilic to hydrophobic. Thus, the encapsulated fluorophores experience a microenvironment change from water-rich to polymer-rich and therefore a change of the polarity and/or viscosity of the microenvironment. This change can significantly increase the quantum yield of the polarity- and viscosity-sensitive fluorophore. As a result, when a T-SFP stays in a temperature below the LCST, its fluorescence is usually weak. When temperature rises over the LCST, the T-SFP can emit strong fluorescence.
In our previous works (6), we summarized five parameters that characterize the performance of a T-SFP. They are: (I) the fluorescence peak excitation and emission wavelengths (λex and λem); (II) the fluorescence intensity ratio between its on and off states (Ion/Ioff); (III) the fluorescence lifetime ratio between its on and off states (τon/τoff); (IV) the temperature threshold to switch on the fluorescence (Tth); and (V) the temperature transition bandwidth (TBW). In this work, we synthesized, characterized, and selected a series of T-SFPs. We adopted two series of NIR fluorophores aza-BODIPY (ADP, we will use ADP for abbreviation in this work) and Zinc phthalocyanine (ZnPc) and four pluronic polymers. Each of fluorophore was encapsulated in one type of pluronic polymer to form temperature-switchable nanoparticles by sonication. The dyes in the ADP and ZnPc families are excellent NIR fluorophores and their quantum efficiencies vary in response to solvents’ polarity and viscosity change (8,9,13,14). The pluronic polymers (i.e., F127, F98, F68, and F38) contain two hydrophilic poly(ethylene oxide) (PEO) and one hydrophobic poly(propylene oxide) (PPO), and they are arranged in a PEO-PPO-PEO triblock structure. When the polymers form nanoparticles, the dye molecules may be more likely encapsulated inside the particles by the hydrophobic part. When the temperature is increased above a threshold, the particle structure may be changed significantly, which makes the dye environment more hydrophobic, less polar and more viscous. Thus, the polarity- and/or viscosity-sensitive dyes experience an increase of the quantum efficiency and the fluorescence emission intensity. By combination of the two series of dyes and the four polymers, we can select the nanoparticles with excellent temperature-switchable fluorescence properties.
We characterized these nanoparticles based on the above mentioned parameters. We also investigated several factors that might change their Tths. These nanoparticles can be potentially used for fluorescence imaging and sensing (15-25). This work has several novelties (1). This work adopted four pluronic polymers (F127, F98, F68, and F38). The four polymers have different lower critical solution temperatures (LCSTs) and thus provide a wide dynamic range of Tth (2). This work recognized two factors that could significantly affect the nanoparticle’s Tths: one is pluronic categories as mentioned in (I), and another is nanoparticles’ concentration. The nanoparticles’ concentration will affect their Tths significantly. Base on (I) and (II), we can synthesize the nanoparticles with a wide range and a precise control of Tths, and this is very useful when they are adopted for temperature sensing/imaging (IV). This work adopted two series of NIR dyes (ADP and ZnPC) for the synthesis and provide different spectral fingerprints for multi-color fluorescence imaging.
Methods
Chemical materials
The ADP fluorophores include BF2-chelated azadipyrromethene (i.e., aza-BODIPY, ADP is used in this study for short. As a note, in the following paragraphs, when we refer ADP as a specific dye, it means azaBODIPY without any additional functional group unlike other three types do.), BF2-chelated 4-{2-[3-(4-hydroxyphenyl)-5-phenyl-1H-pyrrol-2ylimino]-5-phenyl-2H-pyrrol-3-yl}phenol (ADP(OH)2-Top), BF2-chelated [5-(4-hydroxyphenyl)-3-phenyl-1H-pyrrol-2-yl]-[5-(4-hydroxyphenyl)-3-phenylpyrrol-2-ylidene] amine (ADP(OH)2-Bottom), BF2-chelated azadipyrromethene with two cyanocinnamic acid groups (ADP(CA)2). They were synthesized at the Department of Chemistry, University of North Texas (Denton). The details of aza-BODIPY fluorophores can be found in our previous works (8,9,26-28). The ZnPc fluorophores include zinc phthalocyanine (ZnPc), zinc 2, 9, 16, 23-tetra-tert-butyl-29H, 31H-phthalocyanine (ZnttbPc), and zinc 1, 2, 3, 4, 8, 9, 10, 11, 15, 16, 17, 18, 22, 23, 24, 25-hexadecafluoro-29H, 31H-phthalocyanine (ZnHFPc). They were purchased from Sigma-Aldrich Corporate (St. Louis, MO, USA). The details of ZnPc related fluorophores can be found in manufacturer’s datasheet (29). The polymers include pluronics F127, F98, F68, and F38. Tetrabutylammonium iodide (TBAI), pluronic F127, and chloroform was purchased from Sigma-Aldrich Corporate (St. Louis, MO, USA). Pluronic F98, F68, F38 were purchased from BASF Corporation (Vandalia, IL, USA). All chemicals were used as received without further purification.
Synthesis of ADP/ZnPC encapsulated pluronic nanoparticles
The synthesis protocol is from our previous work (8,9). 5 g (or 1 g) pluronic polymer (F127, F98, F68, or F38) was dissolved in 100 mL DI water (pH=8.5) to make 5% (or 1%) pluronic solution. Each of ADP-series fluorophores (ADP, ADP(OH)2-Bottom, ADP(OH)2-Top, or ADP(CA)2) or ZnPc-series fluorophores (ZnPc, ZnttbPc, or ZnHFPc) was selected and encapsulated in the nanoparticles. 0.4 mg ADP fluorophore (or 1.2 mg ZnPc fluorophore) was mixed with 4.8 mg TBAI (cosolvent) and then dissolved in 6 mL chloroform. The fluorophore solution was added dropwise to 15 mL of the pluronic solution, with 600 rpm stirring. The mixture was then under sonication (power =40 Watts) for 4 mins to form fluorophore-encapsulated pluronic-based nanoparticles. The mixture was stirred at 475 rpm in chemical hood overnight until chloroform evaporated thoroughly. The solution was filtered (filter pole size: 450 µm) to purify the sample. All steps were conducted at room temperature. The synthesis protocols are the same for all polymers’ and dyes’ combination.
Fluorescence intensity and lifetime measurement system
The measurement system was developed in our previous work (6). Briefly, the excitation light was from a sub-nanosecond nitrogen-pumped pulsed dye-laser with wavelength centered at 655 nm and pulse width ~0.8 ns. One band-pass interference excitation filter (650/60 nm) was placed in front of the laser output. The nanoparticle sample (volume =3 mL) was placed in a quartz cuvette and submerged in a transparent glass tank filled with water. The water temperature was controlled via a temperature controller. The laser beam illuminated the sample in the cuvette and the emitted fluorescence photons were captured by a photomultiplier tube (PMT) at a 90-degree angle from the laser beam. One band-pass interference emission filter (711/25 nm) was placed in front of the PMT. Finally, the signal was acquired in a multichannel oscilloscope. The fluorescence pulses were recorded at different temperatures. The excitation laser pulse was also measured. We adopted the measured laser pulse as an impulse response function (IRF) of the system. At each temperature, we counted the signal pulse peak as fluorescence intensity. In order to calculate fluorescence lifetime, we de-convolved the normalized fluorescence pulse from IRF and got an exponential decay function. We counted the decay constant as fluorescence lifetime. It is worth mentioning that we measured all the samples’ fluorescence response to a temperature change through conventional heating (i.e., water bath) rather than ultrasound-induced heating, because the purpose of this study is to quantify the thermal properties of these agents.
Characterization of thermal switchable properties of fluorescence from the nanoparticles
As mentioned in the Introduction, there are five parameters that can be used to quantify the thermal switchable properties of the fluorescence from the nanoparticles. Figure 1 shows a diagram indicating some of the characterization parameters. In the plot, the x-axis represents the environment temperature of the nanoparticle, and the y-axis represents the nanoparticle’s fluorescence intensity (or lifetime). The fluorescence intensity (or lifetime) changes as a function of temperature. Typically, the fluorescence intensity (or lifetime) is weak (or short) when the temperature is below the Tth. When the temperature rises above the Tth, the fluorescence intensity (or lifetime) increases significantly. When the temperature rises across the TBW, the fluorescence intensity (or lifetime) becomes stabilized again. The fluorescence intensity (or lifetime) ratio between the on and off states are termed as Ion/Ioff (or τon/τoff). For temperature imaging/sensing, an ideal T-SFP should have (I) NIR λex and λem (which are mainly determined by the fluorophore); (II) high Ion/Ioff and τon/τoff; and (III) adjustable Tth and TBW. NIR wavelengths provide good penetration to biological tissue; high Ion/Ioff and τon/τoff provide high temperature sensitivity; and adjustable Tth and TBW provide an appropriate range of temperature imaging/sensing. Although the reversibility of these nanoparticles is not measured in this work, our previous works based on some nanoparticles used in this study have shown that the nanoparticles can be switched on and off repeatedly for several cycles (8,20).
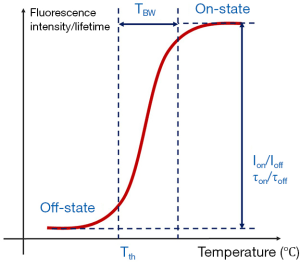
One of the motivations of this work is to evaluate these nanoparticles with different combination of dyes and polymers and select those with excellent temperature-switchable fluorescence property. The characterizations of nanoparticles could be found in our previous work (8,15). In addition, we evaluate the encapsulation efficiency of each nanoparticle based on the its temperature-fluorescence property due to the fact that it is difficult to accurately measure encapsulation efficiency by directly measuring the percentage of dye precipitates (i.e., free dyes outside encapsulation). Those nanoparticles with excellent temperature switchable fluorescence property can be selected for future uses, such as USF imaging, temperature measurement, and other applications.
Results
Fluorescence intensity and lifetime vs. temperature
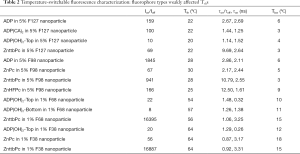
As described in the Methods, we measured the nanoparticles’ fluorescence intensity and lifetime as a function of temperature and characterized the measurement results. Figure 2 shows two examples. In Figure 2A, it shows the measurement of ADP encapsulated in 5% pluronic F98 nanoparticle. It has a high Ion/Ioff =~1,845, and a high τon/τoff =2.86 (τon =2.11 ns), which means both its fluorescence intensity and lifetime increase significantly when the temperature rises over its Tth. Its Tth =~28 °C, and Tbw =~6 °C. Figure 2B show the measurement of ZnttbPc in 1% pluronic F68 nanoparticle. Similarly, it also has a high Ion/Ioff =~16,395. Differently, its fluorescence lifetime slightly changes with a temperature rise. Its τon/τoff =1.06 (τon =3.25 ns). Its Tth =~56 °C, and Tbw =~15 °C. Based on these two examples, we found their Ion/Ioff, Tth, τon/τoff, and Tbw varied. Their characterizations were also presented in Table 1. In this work, we synthesized all the nanoparticles based on different combinations of fluorophore categories and pluronic categories as well as pluronic concentrations. Then, we characterized and selected these nanoparticles based on their thermos-switchable properties of fluorescence, from three perspectives: (I) those with a high Ion/Ioff; (II) those with a high τon/τoff; and (III) those with various Tths. Note that some nanoparticles didn’t show a thermal-switchable property (i.e., a low Ion/Ioff and/or τon/τoff) and were excluded. After selection, we studied several factors that affected the nanoparticles’ Tths. The results were presented and discussed in the next sections.


Full table
Temperature threshold vs. fluorophores
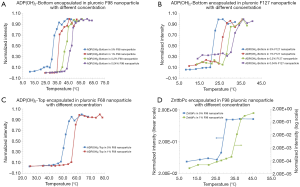
In this section, we studied the variation of Tths. Four polymer solutions were prepared based on different types of pluronics and concentrations: (I) 5% F127; (II) 5% F98; (III) 1% F68; and (IV) 1% F38. In each polymer solution, different fluorophores were encapsulated and the nanoparticles were synthesized and characterized. In 5% F127 nanoparticle, four fluorophores [i.e., ADP, ADP(CA)2, ADP(OH)2-Bottom, and ZnttbPc] were encapsulated. Figure 3A shows their normalized fluorescence intensity as a function of temperature. Their Tths were ~22, 22, 20, and 22 °C, respectively. It shows their Tths were very close to each other (~2 °C difference). Likewise, Figure 3B shows the normalized fluorescence intensity as a function of temperature of four samples: ADP in 5% F98 nanoparticle, ZnPc in 5% F98 nanoparticle, ZnttbPc in 5% F98 nanoparticle, and ZnHFPc in 5% F98 nanoparticle. Their Tths were ~28, 30, 28, and 25 °C, respectively. Also, their Tths were close to each other (≤5 °C). Similar results were also presented when different dyes were encapsulated in 1% F68 nanoparticles (Figure 3C) or 1% F38 nanoparticles (Figure 3D). The Tths were close to each other (≤5 °C) when the nanoparticles had the same type of pluronic and the same concentration. All the nanoparticles showed excellent thermal-switchable properties of fluorescence (high Ion/Ioff and/or τon/τoff). Their characterization results were summarized in Table 2. In this section, we concluded that the fluorophore type weakly affected the nancapsules’ Tths (Tth difference ≤2–5 °C), when nanoparticles were synthesized based on the same type of pluronic and concentration.
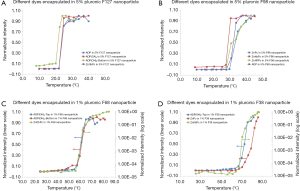
Full table
Temperature threshold vs. pluronic types
Figure 3 also indicated that the nanoparticles’ Tths varied significantly when using different types of pluronics. In this section, we studied this effect in details. We encapsulated the same fluorophore in nanoparticles using different pluronics. The nanoparticles were characterized and compared with each other. Figure 4A shows the characterization results of four samples: ADP(OH)2-Top in 1% F127 nanoparticle, ADP(OH)2-Top in 1% F98 nanoparticle, ADP(OH)2-Top in 1% F68 nanoparticle, ADP(OH)2-Top in 1% F38 nanoparticle. Based on the normalized fluorescence intensities as a function of temperature, their Tths were ~24, 34, 54, and 64 °C, respectively. Since the same fluorophore (i.e., ADP(OH)2-Top) and pluronic concentration were adopted, the major factor that leaded to the change of the nanoparticles’ Tths is the types of the pluronics. Figure 4B,C,D shows the similar results when other fluorophores were used (i.e., ZnttbPc in Figure 4B, ADP in Figure 4C, and ZnPc in Figure 4D). All the nanoparticles in Figure 4 show excellent thermal-switchable properties and their characterization results were summarized in Table 3. These examples indicate that the Tths were significantly affected by the types of pluronics. It is worth mentioning that, in these plots, some curves were presented in different scales (linear or log scale) for a better visualization, due to that the nanoparticles’ Ion/Ioffs varied significantly in scale (from tens to ten thousands of folds). Based on the results in Figure 4, we conclude that the type of pluronics is a main factor that significantly changes the Tths of nanoparticles. Briefly, F127-based nanoparticle provided the lowest Tth and F38-based nanoparticle provided the highest. The pluronics’ Tth sequence is F127 < F98 < F68 < F38. This is because that the Tths of nanoparticles were mainly related to the LCSTs of the pluronics.

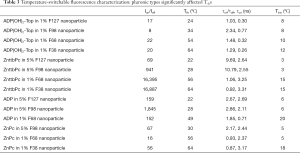
Full table
Temperature threshold vs. nanoparticle concentrations
In this section, we studied another factor: the concentrations of the nanoparticles, which also affected the Tths. The nanoparticle solution was diluted to different concentrations and each solution was characterized. The concentration of each nanoparticle solution was expressed by the pluronic concentration. For example, we selected ADP(OH)2-Bottom in 5% F98 nanoparticle as the original sample and diluted it by 5, 25, and 125 times correspondingly. Thus, we obtained ADP(OH)2-Bottom in 1%, 0.2% and 0.04% F98 nanoparticle, respectively. The characterization of all the nanoparticles in the section were summarized in Table 4. Figure 5A shows their normalized fluorescence intensity as a function of temperature. Their Tths were ~28, 34, 37, and 41 °C, respectively. The result indicates that when the nanoparticles were diluted to a lower concentration, the Tth shifted to the higher temperature. Figure 5B,C,D respectively show the similar measurement results of the other three nanoparticle samples: ADP(OH)2-Bottom in F127 nanoparticle, ADP(OH)2-Top in F68 nanoparticle, and ZnttbPc in F98 nanoparticle. When each sample was diluted to a lower concentration, its Tths increased correspondingly. Thus, we concluded that the nanoparticle’s Tths increased with the decrease of the concentration for the adopted fluorophores and pluronics.

Full table
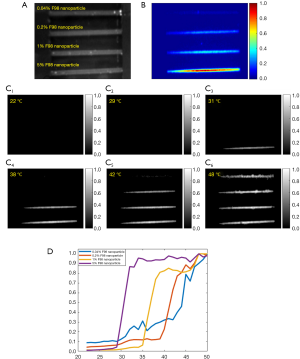
Observation of different temperature-switching thresholds of nanoparticles under a camera
We respectively injected four nanoparticle samples with different temperature thresholds into four silicone tubes and merged tubes into a water bath whose temperature was controlled via the same method discussed before. The dynamic fluorescence change of the samples vs. temperature was simultaneously monitored via a camera. The silicone tubes have an inner diameter of 760 µm. A 671 nm laser was adopted as the excitation light. An intensified charge-coupled device (ICCD) camera was placed on the top for fluorescence imaging of the four tubes. Four 830 nm long-pass interference filters and one 830 nm long-pass absorption filter were placed in front of the camera lens as emission filters. The water temperature was increased gradually from room temperature =22 °C to a high temperature =50 °C. At different temperatures (step size: 1 °C), the ICCD camera recorded the fluorescence image of all the tubes. As an example, we imaged the samples of ADP(OH)2-Bottom encapsulated in pluronic F98 with a concentration of 5%, 1%, 0.2% and 0.04%, which correspond to different temperature thresholds. Figure 6A shows a white image of the four tubes under the camera. From the bottom to top, the sample concentration is 5%, 1%, 0.2% and 0.04%. Figure 6B shows the normalized background fluorescence image of the tubes at room temperature =22 °C when all the four nanoparticles are at their off states. Due to the dilution, the background fluorescence decreases with the reduction of the nanoparticle concentration. Note that the tube at the bottom has the highest nanoparticle concentration and therefore it shows the most obvious background signal. Background fluorescence signal is usually caused by a fact that the fluorophores in the nanoparticles are not completely switched off even though they are at off states. Usually, we call this background signal as non-100%-off background signal. In order to visualize the four tubes in the same figure, we individually normalized the fluorescence signal for each tube. Figure 6C show the corresponding results. The images show their fluorescence intensities at the temperatures of 22, 29, 31, 38, 42 and 48 °C, respectively. A displaying threshold of 0.25 was applied for each image so that the background fluorescence can be removed. Clearly, when the temperature was 22 and 29 °C (Figure 6C1,C2), all the fluorescence tubes were at “off” state, which indicated that the two temperatures were below the Tths of all samples. When the temperature rose to 31 °C (Figure 6C3), the first bottom tube [filled with ADP(OH)2-Bottom encapsulated in 5% pluronic F98 nanoparticles] was switched “on”. When the temperature was 38 °C (Figure 6C4), the second bottom tube [filled with ADP(OH)2-Bottom encapsulated in 1% pluronic F98 nanoparticles] was switched “on”. When the temperature was 42 °C (Figure 6C5), the third bottom tube [filled with ADP(OH)2-Bottom encapsulated in 0.2% pluronic F98 nanoparticles] was switched “on”. Finally, when the temperature rose to 48 °C (Figure 6C6), all the tubes were switched “on”. Figure 6D shows the normalized fluorescence intensity in each tube plotted as a function of temperature. The measurement results were similar to that in Figure 5A. This experiment clearly shows that the measured Tths of nanoparticles in our previous system (6) is reliable and also repeatable under the camera-based fluorescence imaging system.
Discussion
The nanoparticles are appropriate for ultrasound-switchable fluorescence (USF) imaging
Some pluronic nanoparticles have been selected and adopted as USF contrast agents in our previous work (8,15-17). They have several advantages in USF imaging. First, the fluorophores (i.e., ADP or ZnPC series) are NIR dyes so their fluorescence can penetrate deeper biological tissue compared with light with visible wavelengths. Second, compared with other USF contrast agents, many nanoparticles (i.e., ADP in 5% F98 nanoparticle) show a high Ion/Ioff, which helps quench the background fluorescence to reduce background noise and further improve signal-to-noise ratio (SNR) in USF image. Third, some nanoparticles (i.e., ZnttbPc in 5% F98 nanoparticle) also showed a high τon/τoff. A high τon/τoff could help increase the SNR of a USF image when using a time-domain USF imaging system (20). In conclusion, the nanoparticles have excellent thermal-switchable properties and are appropriate contrast agents for USF imaging.
The nanoparticles are appropriate for temperature sensing
In this work, we demonstrated that the nanoparticles’ Tths varied in a wide temperature range from ~20 to ~64 °C. Their Tths could be adjusted by changing the pluronic categories and/or the nanoparticle concentrations. These nanoparticles usually had an excellent fluorescence intensity switching property (i.e., a high Ion/Ioff) so that they should have very high temperature sensitivity. Also, these nanoparticles had a TBW of a few to tens of Celsius degrees (from 3 up to 20 °C). Thus, the nanoparticles can be used as temperature sensors. Their temperature sensing ranges depend on the TBW: when the temperature crosses the TBW, the fluorescence increases significantly. For example, the ADP in 5% F98 nanoparticle has a fluorescence intensity increase most when the temperature rises from ~28 to ~34 °C (seen in Figure 2A), which means this nanoparticle has the most efficient temperature sensing range from 28 to 34 °C. By combining these nanoparticles with different Tths and TBWs, we can develop temperature sensors covering a wide temperature range. Further work about this application will be explored in our future work.
Conclusions
In this work, we successfully synthesized, characterized, and selected a series of NIR nanoparticles, by encapsulating two series of NIR fluorophores (ADP and ZnPc) in four pluronic polymers (F127, F98, F68, and F38). These nanoparticles showed excellent temperature-switchable properties of fluorescence intensity and/or lifetime. Also, we investigated some factors (i.e., pluronic categories and nanoparticles’ concentration) that significantly affected the nanoparticles’ Tths while other (i.e., fluorophore categories) that weakly affected Tths. By selecting appropriate pluronic categories and adjusting the nanoparticle’s concentration, we can synthesize the nanoparticles with a wide range of Tths. These temperature-switchable fluorescence nanoparticles can be used for biomedical imaging (such as tissue fluorescence and/or USF imaging) and in vivo tissue temperature sensing/imaging.
Acknowledgments
Thanks for Dr. Kytai Nguyen and Dr. Yi Hong for allowing us to use some equipment in their labs to synthesize and characterize contrast agents. Thanks for Dr. Francis D’Souza for providing the ADP series fluorophores.
Funding: This work was supported in part by funding from Cancer Prevention & Research Institute of Texas (CPRIT) RP170564 (Baohong Yuan).
Footnote
Provenance and Peer Review: With the arrangement by the Guest Editors and the editorial office, this article has been reviewed by external peers.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-797). The special issue “Advanced Optical Imaging in Biomedicine” was commissioned by the editorial office without any funding or sponsorship. SY reports grants from Cancer Prevention & Research Institute of Texas, during the conduct of the study; in addition, Dr. SY has a patent Systems and Methods for Thermometry and Theranostic Applications pending. ZW reports grants from Cancer Prevention & Research Institute of Texas, during the conduct of the study. TY reports grants from Cancer Prevention & Research Institute of Texas, during the conduct of the study; in addition, Dr. TY has a patent Systems and Methods for Thermometry and Theranostic Applications pending, a patent Ultrasound-switchable fluorescence imaging having improved imaging speed pending, and a patent Significantly improving sensitivity of ultrasound-switchable fluorescence technique for high-resolution, deep tissue, and dynamic imaging pending. BY reports grants from Cancer Prevention & Research Institute of Texas, during the conduct of the study; in addition, Dr. BY has a patent Systems and Methods for high-resolution imaging issued, a patent Systems and Methods for Thermometry and Theranostic Applications pending, a patent Highly specific tissue imaging pending, a patent Multiple biomarkers imaging for high specificity pending, a patent Ultrasound-switchable fluorescence imaging having improved imaging speed pending, a patent Systems and Methods for surgical guidance in breast cancer surgery and lymph node dissection pending.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626-34. [Crossref] [PubMed]
- Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng 2017;1:0010.
- Terai T, Nagano T. Fluorescent probes for bioimaging applications. Curr Opin Chem Biol 2008;12:515-21. [Crossref] [PubMed]
- Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev 2010;110:2620-40. [Crossref] [PubMed]
- Guo Z, Park S, Yoon J, Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev 2014;43:16-29. [Crossref] [PubMed]
- Cheng B, Wei MY, Liu Y, Pitta H, Xie Z, Hong Y, Nguyen KT, Yuan B. Development of Ultrasound-switchable Fluorescence Imaging Contrast Agents based on Thermosensitive Polymers and Nanoparticles. IEEE J Sel Top Quantum Electron 2014;20:6801214. [PubMed]
- Yu S, Cheng B, Yao T, Xu C, Nguyen KT, Hong Y, Yuan B. New generation ICG-based contrast agents for ultrasound-switchable fluorescence imaging. Sci Rep 2016;6:35942. [Crossref] [PubMed]
- Cheng B, Bandi V, Yu S, D'Souza F, Nguyen KT, Hong Y, Tang L, Yuan B. The Mechanisms and Biomedical Applications of an NIR BODIPY-Based Switchable Fluorescent Probe. Int J Mol Sci 2017;18:384. [Crossref] [PubMed]
- Saremi B, Bandi V, Kazemi S, Hong Y, D'Souza F, Yuan B. Exploring NIR Aza-BODIPY-Based Polarity Sensitive Probes with ON-and-OFF Fluorescence Switching in Pluronic Nanoparticles. Polymers (Basel) 2020;12:540. [Crossref] [PubMed]
- Cui J, Kwon JE, Kim HJ, Whang DR, Park SY. Smart Fluorescent Nanoparticles in Water Showing Temperature-Dependent Ratiometric Fluorescence Color Change. ACS Appl Mater Interfaces 2017;9:2883-90. [Crossref] [PubMed]
- Zhao Y, Shi C, Yang X, Shen B, Sun Y, Chen Y, Xu X, Sun H, Yu K, Yang B, Lin Q. pH- and Temperature-Sensitive Hydrogel Nanoparticles with Dual Photoluminescence for Bioprobes. ACS Nano 2016;10:5856-63. [Crossref] [PubMed]
- Cheng CC, Liao ZS, Huang JJ, Lee DJ, Chen JL. Supramolecular polymer micelles as universal tools for constructing high-performance fluorescent nanoparticles. Dyes and Pigments 2017;137:284-292. [Crossref]
- Kakade S, Ghosh R, Palit DK. Excited State Dynamics of Zinc–Phthalocyanine Nanoaggregates in Strong Hydrogen Bonding Solvents. J Phys Chem C 2012;116:15155-66. [Crossref]
- Gümrükçü G, Karaoğlan GK, Erdoğmuş A, Gül A, Avcıata U. Photophysical, photochemical, and BQ quenching properties of zinc phthalocyanines with fused or interrupted extended conjugation. J Chem 2014;2014:435834. [Crossref]
- Cheng B, Bandi V, Wei MY, Pei Y, D'Souza F, Nguyen KT, Hong Y, Yuan B. High-Resolution Ultrasound-Switchable Fluorescence Imaging in Centimeter-Deep Tissue Phantoms with High Signal-To-Noise Ratio and High Sensitivity via Novel Contrast Agents. PLoS One 2016;11:e0165963. [Crossref] [PubMed]
- Kandukuri J, Yu S, Cheng B, Bandi V, D'Souza F, Nguyen KT, Hong Y, Yuan B. A Dual-Modality System for Both Multi-Color Ultrasound-Switchable Fluorescence and Ultrasound Imaging. Int J Mol Sci 2017;18:323. [Crossref] [PubMed]
- Kandukuri J, Yu S, Yao T, Yuan B. Modulation of ultrasound-switchable fluorescence for improving signal-to-noise ratio. J Biomed Opt 2017;22:76021. [Crossref] [PubMed]
- Yao T, Yu S, Liu Y, Yuan B. Ultrasound-Switchable Fluorescence Imaging via an EMCCD Camera and a Z-Scan Method. IEEE J Sel Top Quantum Electron 2019;25:7102108. [Crossref]
- Yao T, Yu S, Liu Y, Yuan B. In vivo ultrasound-switchable fluorescence imaging. Sci Rep 2019;9:9855. [Crossref] [PubMed]
- Yu S, Yao T, Yuan B. An ICCD camera-based time-domain ultrasound-switchable fluorescence imaging system. Sci Rep 2019;9:10552. [Crossref] [PubMed]
- Liu Y, Yao T, Cai W, Yu S, Hong Y, Nguyen KT, Yuan B. A Biocompatible and Near-Infrared Liposome for In Vivo Ultrasound-Switchable Fluorescence Imaging. Adv Healthc Mater 2020;9:e1901457. [Crossref] [PubMed]
- Yu S, Yao T, Liu Y, Yuan B. In vivo ultrasound-switchable fluorescence imaging using a camera-based system. Biomed Opt Express 2020;11:1517-38. [Crossref] [PubMed]
- Pandey N, Menon JU, Takahashi M, Hsieh JT, Yang J, Nguyen KT, Wadajkar AS. Thermo-responsive Fluorescent Nanoparticles for Multimodal Imaging and Treatment of Cancers. Nanotheranostics 2020;4:1-13. [Crossref] [PubMed]
- Wang Z, Yong TY, Wan J, Li ZH, Zhao H, Zhao Y, Gan L, Yang XL, Xu HB, Zhang C. Temperature-sensitive fluorescent organic nanoparticles with aggregation-induced emission for long-term cellular tracing. ACS Appl Mater Interfaces 2015;7:3420-5. [Crossref] [PubMed]
- Julià López A, Ruiz‐Molina D, Landfester K, Bannwarth MB, Roscini C. Off/On Fluorescent Nanoparticles for Tunable High‐Temperature Threshold Sensing. Adv Funct Mater 2018;28:1801492. [Crossref]
- Amin AN, El-Khouly ME, Subbaiyan NK, Zandler ME, Supur M, Fukuzumi S, D'Souza F. Syntheses, electrochemistry, and photodynamics of ferrocene-azadipyrromethane donor--acceptor dyads and triads. J Phys Chem A 2011;115:9810-9. [Crossref] [PubMed]
- Bandi V, El-Khouly ME, Ohkubo K, Nesterov VN, Zandler ME, Fukuzumi S, D'Souza F. Excitation-wavelength-dependent, ultrafast photoinduced electron transfer in bisferrocene/BF2-chelated-azadipyrromethene/fullerene tetrads. Chemistry 2013;19:7221-30. [Crossref] [PubMed]
- Bandi V, El-Khouly ME, Nesterov VN, Karr PA, Fukuzumi S, D’Souza F. Self-assembled via metal–ligand coordination azaBODIPY–zinc phthalocyanine and azaBODIPY–zinc naphthalocyanine conjugates: synthesis, structure, and photoinduced electron transfer. J Phys Chem C 2013;117:5638-49. [Crossref]
- Zinc phthalocyanine, Dye content 97%. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/24860701


