Imaging endocervical mucus anatomy and dynamics in macaque female reproductive track using optical coherence tomography
Introduction
Human immunodeficiency virus (HIV) infection and its consequent disease, acquired immunodeficiency syndrome (AIDS), affect around 40 million people worldwide (1). While heterosexual transmission accounts for about 80% of the incidents, more than half of the victims are female (2). The anatomic structure of the female reproductive tract (FRT) makes them more prone to HIV infections. Furthermore, the low social status of women, cultural and sexual norms in certain regions all pose female at higher risk (3,4). Combined with the long latent period, highly infectious during all stages, and no existing cure, all these conditions pose it as one of the most lethal disease for woman worldwide (1). As the immune system is weakened, victims are vulnerable to other infections and need intensive medical attention, which bring huge burden on both economic and social well-being of the affected area (5).
Despite the current methods using exogenous substances to prevent AIDS infection (e.g., vaginal barrier devices and antibiotics) (6-8), there are more and more investigations focus on the intrinsic AIDS defending systems. Among them, endocervical mucus serves as an important barrier. Consisting of various glycoprotein and antibodies, the normal mucus is very effective at trapping and neutralizing invading infectious microbes (9). It is reported that mucus, together with other defending mechanisms, can lower the incidence of HIV infection to 0.0001-0.0040 per sexual act (2,10). Disruptions caused by the presence of ulcerative sexually transmitted diseases (STDs), including herpes simplex virus-2 (HSV-2) infection, cancroid and syphilis, will greatly weaken the effect of this natural barrier (11). These complexities will affect the viscosity and thickness of the secreted mucus layer, change of both will influence how fast pathogens penetrate and reach epithelial cells and infect the host (11-13). Therefore, a widely-accessible method to evaluate the integrity of the barrier will provide an effective screening for those at higher risk of HIV infection. For example, female with lower than normal mucus thickness will be at higher risk of infection, thus should be given higher priority when considering preventive medication.
One critical parameter indicating the integrity of the endocervical barrier is the mucus thickness, which is still challenging to monitor to date, partially because its gel-like appearance prevents direct measurement by visual inspection. In this study we propose to use optical coherence tomography (OCT) to dynamically measure the mucus thickness in vivo. OCT is an optical imaging modality that generates 3-dimensional mapping of scattering contrast from the sample. Our current OCT system can achieve ~12 µm lateral resolution and micrometer-scale depth resolution. The mucus contains intrinsic contrast originating from the cell debris and undissolvable substance, whose characteristic back-scattering pattern differentiates it from underlying tissue, allowing quantitative measurement of its thickness. We demonstrate here that OCT is capable of visualizing and performing endocervical mucus thickness measurements ex vivo. We also achieve real-time dynamic monitoring of mucus secreting and hydrolysis. Finally, we integrate our OCT system into a miniature sized endoscopic probe that can be easily inserted into macaque FRT and potentially allows in vivo imaging on endocervical lumen.
Methods and materials
Table-top OCT imaging system
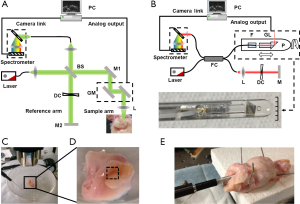
We used a spectral-domain OCT system working at the visible-light spectral range (vis-OCT) to image the macaque endocervical sample (Figure 1A). The detailed description of the system can be found in our earlier publication (14). Briefly, a super continuum laser (SuperK, NKT photonics) provided illuminating light ranging from 512 to 620 nm. The light was split by a 50:50 beam splitter (BS) into the sampling arm and the reference arm. A pair of galvanometer mirrors (GM) raster scanned the sampling laser beam through a scanning objective (Thorlabs, EFL =37 mm) to cover a rectangular field of view. The back-scattered sample beam and the reference beam were recombined, and a home-built spectrometer captured the spectral interference pattern generated. When set at 25 kHz A-line rate, it took 2.6 seconds to acquire an OCT volume consisting of 65,536 A-lines, a typical value used in our experiment.
Endoscopic OCT probe
To demonstrate that our approach has the ability to perform in vivo measurements, we constructed a prototype endoscopic OCT probe that can perform linear and circular scans. To achieve higher penetration depth for in situ measurement, near infrared (NIR) light source was used instead of the visible light. The endoscopic probe is a fiber-based, miniature sized lens-prism complex. The schematic diagram of the OCT probe was shown in Figure 1B. We used a gradient-index (GRIN) lens to obtain light focusing. A right-angle prism is attached on the GRIN lens to achieve desired side-view imaging. The lens-prism complex is mounted on a rotating shaft, which is driven by a step motor to control the circular scan. A motorized linear translation stage was used to move the probe from the proximal to distal position, allowing a 3-dimensional cylindrical scanning pattern to be performed. The photo in Figure 1B shows the dimension of a finished prototype endoscopic OCT probe. The outer diameter of the probe is roughly 4.5 mm, which can be easily inserted into the macaque FRT.
Tissue preparation and imaging process
Endocervical and vaginal tissue samples were harvested from sacrificed macaque. The samples were kept refrigerated in PBS solution for transportation and storage, which happened in less than 24 hours. For ex vivo dynamic monitoring of mucus thickness, we dissected and trimmed the sample into pieces of 0.5 by 0.5 inch to expose the endocervical duct. Tissue was incubated in a homemade imaging-compatible incubator with the temperature set to 37 °C. The incubator contained 1% PBS solution to maintain the moisture and osmolality (Figure 1C).
In order to imaging the dynamic secretion of endocervical mucus, we gently flushed away any remaining mucus with PBS on our sample prior to the imaging sequence and took OCT imaging immediately thereafter. The imaging area was indicated by a dashed box in Figure 1D, which covered an area of 2×2 mm2. After the initial imaging, we continue to take OCT images at the same location every 10 minutes to monitor the thickness change of the mucus layer for 40 minutes. During the entire imaging period, the sample was half submerged in PBS solution and kept at constant temperature of 37 °C.
After imaging the secretion sequence, we performed a dynamic monitoring of mucus hydrolysis. We used neuraminidase to hydrolyze the terminal α-Neu5Ac of mucus glycoprotein. We re-suspended 10,000 units of neuraminidase (50,000 units/mL, New England BioLabs) in 500 µL-50 mM sodium citrate solution. The pH of the reactive solution was controlled to be 6.0 and incubated at 37 °C for 5 minutes prior to use. We applied the neuraminidase reactive solution drop by drop using a disposable pipet until the entire exposed surface of the sample was covered, which took about 2.5 minutes. We immediately took an OCT image after we applied neuraminidase. The same imaging protocol as described above was used. After that, we took another OCT image 2.5 minutes later and three more every 5 minutes. We applied a few drops of neuraminidase every other minute during the imagining sequence to replenish the consumed enzyme.
For ex vivo endoscopic imaging on vagina duct, we carefully fixed the intact macaque FRT on a home-made animal dissection table while keeping the vagina opening exposed. We inserted our endoscopic OCT probe into the vagina duct about 1 to 2 inches deep and began 3-dimensional cylindrical imaging (Figure 1E). During imaging acquisition, PBS solution was added to prevent dehydration.
OCT image processing and mucus thickness measurement
To reconstruct the OCT image, we first removed the DC component and normalize the interference spectrum by wavelength-depended light source intensity. We then resampled the spectrum into equal-interval k-space. We applied fast Fourier transform on the re-sampled spectrum to retrieve the depth-resolved OCT structural image. For endoscopic OCT image, a coordinate transformation was performed to map the acquired matrix back to polar form for better visualization.
In order to quantify the dynamic change in mucus layer thickness, we measured the thickest portion of mucus layer in each acquired OCT image stack. Five measurements were taken around the region, and the mean values and standard deviations of the measurements were calculated. We performed 2-sample Student’s t-test to analyze potential changes in the mucus layer thickness.
Results
Dynamic monitoring of mucus secretion and hydrolysis
Figure 2A shows a cross sectional B-scan frame using the bench top OCT system. We identified the typical endocervical tissue structures including epithelium and lamina propria (LP) in OCT images. Mucus was recognized by a strong reflection from mucus surface and scattered cell debris within mucus.
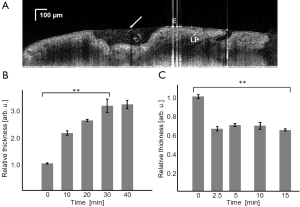
The relative mucus thickness change when cultured with PBS was plotted in Figure 2B. During the imaging sequence, we observed a nearly linear increase of mucus thickness within the first 30 minutes of the procedure with up to threefold thickness. The change in the thickness was statistically significant for these time points (P<0.01). The mucus thickness reached a plateau thereafter. We continued to make one additional measurement but no statistically significant change was observed.
After applying neuraminidase reactive solution, we observed an immediate decrease in mucus thickness as shown in Figure 2C. This is characterized by a sudden drop of mucus thickness to about 60% its original value at the 2.5 minutes time point. The drop in the thickness was statistically significant (P<0.01). We did not observe any further change during the 15 minutes observing period.
Endoscopic OCT imaging of intact vaginal duct and mucus
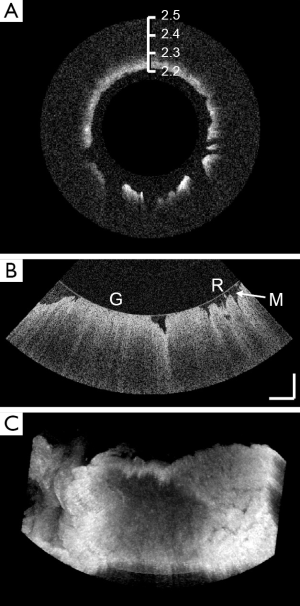
We performed a 360° cylindrical scan of the intact macaque vagina duct ex vivo. Figure 3A shows one of the rotational B-scan images of the entire 3-dimensional volumetric dataset. The image was rescaled to enlarge the tissue layers for better visualization. From this image, we can appreciate the rough surface of the vaginal mucosa. We also conducted a high-resolution volumetric scan covering a scanning angle of 45° (corresponds to 1.2 mm circumference) and 0.8 mm longitudinal displacement. Figure 3B shows one of the B-scan of the volumetric data. Besides some of the flattened surface caused by the pressure asserted by the protective glass shell, we were able to recognize anatomical structures such as vagina rugae. In addition, the lightened granular pattern in the recess of epithelium indicated the presence of vaginal mucus. The entire 3-dimensional volume was rendered in Figure 3C, demonstrating the roughness of inner vagina wall.
Discussion
In the presented study, we demonstrated that OCT can dynamically monitor the thickness of endocervical mucus. We monitored both the secretion and hydrolysis of mucus, characterized by increase and decrease of mucus layer thickness. During the mucus secretion period, we first observed a fast linear increase in mucus layer thickness and then followed by a plateau period. The exact physiological cause of the plateau period remains unclear. One possible explanation is that the gel-like mucus cannot support its weight under gravity, and it reaches its maximum thickness after 30 minutes. During the mucus hydrolysis experiment, we observed a sudden decrease of mucus thickness to about 60% of its original value, and no further change was observed. The results indicated that the neuraminidase used was extremely effective at hydrolyzing mucus glycoproteins. The gel structure may be disrupted instantaneously upon application. We also found that the hydrolyzed residue of mucus may interfere with the measurement, as they still contained undissolvable scattering particles and had characteristic OCT signal similar to mucus. However, this problem can be easily solved by change the orientation of the sample, so the hydrolyzed mucus residue will spontaneously flow away due to gravity.
We also demonstrated that the prototype endoscopic probe can image the intact vagina duct and mucus ex vivo. We were not able to image the canal of cervix due to the size of our probe. Either using a smaller endoscopic probe or applying drugs that dilate endocervical canal should allow the imaging through canal of cervix. Nevertheless, we demonstrated that the designed endoscope probe was capable of acquire a 360° cylindrical OCT image of the inner wall of a tubular hollow organ with the potential of in vivo applications.
Acknowledgements
We are grateful for the generous financial support from the National Science Foundation grants DBI-1353952 and CBET-1055379, and the National Institutes of Health grants R01EY019951, R24EY022883 to HFZ, and R01AI094595 to TJH.
Disclosure: The authors declare no conflict of interest.
References
- Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science 2005;308:1582-3. [PubMed]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 2003;1:25-34. [PubMed]
- D. o. S. Prevention, “Sexually Transmitted Disease Surveillance 2012,” (2014). Available online: http://www.cdc.gov/std/stats12/Surv2012.pdf
- Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: findings of a South African cross-sectional study. Soc Sci Med 2003;56:125-34. [PubMed]
- Haacker M. HIV/AIDS: The Impact on the Social Fabric and the Economy. In: Haacker M. eds. The Macroeconomics of HIV/AIDS. Washington DC: International Monetary Fund, 2004.
- Moench TR, Chipato T, Padian NS. Preventing disease by protecting the cervix: the unexplored promise of internal vaginal barrier devices. AIDS 2001;15:1595-602. [PubMed]
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399-410. [PubMed]
- Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep 2011;8:62-72. [PubMed]
- Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One 2013;8:e76176. [PubMed]
- Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol 2009;83:11196-200. [PubMed]
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75:3-17. [PubMed]
- Røttingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis 2001;28:579-97. [PubMed]
- Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33-42. [PubMed]
- Yi J, Chen S, Backman V, Zhang HF. In vivo functional microangiography by visible-light optical coherence tomography. Biomed Opt Express 2014;5:3603-12. [PubMed]


