
Image-localized body surface marking for the intraoperative localization of pulmonary ground-glass nodules
Introduction
Video-assisted thoracoscopic surgery (VATS) has been established as a standard procedure for the resection of small pulmonary nodules (1). However, ground-glass nodules (GGNs) are thoracoscopically undetectable and impalpable with VATS, therefore requiring accurate and effective preoperative or intraoperative localization techniques for successful tumor excision. The invasive, preoperative localization methods play a vital role in the treatment of pulmonary GGNs. These widely used methods include hook wire (2), medical adhesive (3), radiotracer-guided localization (4), dye localization (5,6), contrast medium injection (7,8) and micro-coil localization (9). Adverse effects of localization and surgery have been commonly reported, such as discomfort, tension, and complications, including minor pneumothorax (7.5–40%), lung parenchyma hemorrhage (13.9–36%), and subcutaneous emphysema (5%) (10-13). Furthermore, dislodgements have been reported at a rate of 2.4–6.9% (10-13) in transit to the operating room (14).
Intraoperative localization methods, including ultrasound localization (15), near-infrared imaging (16), and tactile pressure induction localization (17), are performed under general anesthesia. However, these procedures mandate very specific criteria for patient eligibility, are equipment-dependent, and require lengthy positioning durations, limiting their application in surgery. Based on CT-guided hook wire technology, we examined the viability of marking the body surface by CT before performing puncture positioning during surgery. Our study reports on a set of image-localized body surface marking intraoperative (IBMI) localization approach routinely applied in our practice. We detailed this method and investigated the application of IBMI localization during VATS for pulmonary GGNs. Our findings document the benefits of this non-invasive intraoperative localization procedure, supporting the concept of this approach as the method of choice.
Methods
This study was approved and supervised by the Medical Ethics Committee of the First People’s Hospital of Huzhou. Every patient signed the informed consent form voluntarily before surgery. We performed IBMI localization in 76 patients with lesions between January 2018 and March 2019. IBMI localization approaches were applied to GGNs, due to their difficulty in detection via physical examination, compared to solid tumors. Seventy-six patients (41 women and 35 men, mean age 63.7±8.6, range, 31 to 78) with 76 GGNs [17 pure GGNs (pGGNs), 59 part-solid GGNs (psGGNs)] were included in the study. CT-guided hook wire procedures were conducted in 26 patients before surgery to ensure that this method was feasible, followed by IBMI localizations during the operation. Another 50 patients underwent an IBMI localization directly. The general clinical data, postoperative pathology, depth and size of pulmonary nodules, distance from the pulmonary nodules to the hook wire, distance from the pulmonary nodules to the coagulation point, duration of localization and resection lesions were recorded.
Inclusion criteria: (I) patients who underwent a CT-guided hook wire localization approach displayed no apparent macrovessels, pulmonary vesicles, or other essential tissue structures in the proposed puncture path. (II) Normal blood coagulation function, and high tolerance to thoracoscopic surgery. (III) The patient was over 18 years of age. (IV) The maximum diameter of pulmonary nodules was ≤30 mm by high-resolution CT (HRCT). (V) Pulmonary nodules are identified as GGNs by HRCT.
Exclusion criteria: (I) patients required direct lobectomy. (II) A GGN diameter of >30 mm by HRCT. (III) The patient could not tolerate surgery or had distant metastasis. (IV) GGN nodules located in the middle zone near the hilum of the lung. (V) Distance from GGN to the pleural surface distance of >3.0 cm.
Computed tomography-guided hook wire location method
The initial chest CT scan was used to determine the optimal puncture site, angle, and depth. The puncture route did not traverse important vessels, including the internal mammary, axillary, subclavian, intercostal vessel, and pulmonary vessels. Bulla and interlobar were also avoided to prevent pneumothorax. After applying local anesthesia (2% lidocaine) to the puncture site, the hook wire guide was inserted into the chest wall with the tip close to the parietal pleura. The guide was oriented almost perpendicular to the pleural surface. After verification of the correct plane and angulation, the guide was extended into the lung parenchyma towards the lesion, without penetration to avoid tumor seeding via a puncture tract. The hook wire was then released via the guide.
IBMI location method
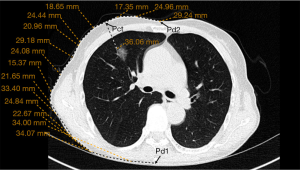
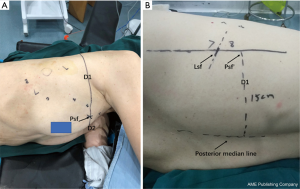
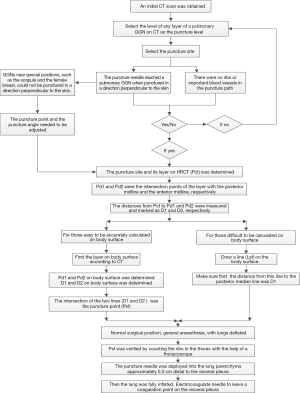
Preoperative procedures: (I) an initial CT scan during the maximum inspiratory phase was obtained. The puncture level was determined by the presence of the pulmonary nodule. The puncture needle was inserted perpendicular to the skin before extending to the pulmonary nodule, ensuring that ribs or important blood vessels were absent from the puncture path. In cases were pulmonary nodules were near exceptional positions, such as the scapula and the female breast, the puncture point, and angle were adjusted if it could not be perpendicular to the skin. Puncture levels were also changed if ribs or major vessels were found to be in the puncture path. (II) The intercostal space of the anterior and posterior median lines was measured and recorded at the level of Pct to determine the puncture site on HRCT (Pct). Pd1 and Pd2 were the intersection points of the layer with the posterior and the anterior midline, respectively. The distances from Pct to Pd1 and Pd2 were measured and marked as D1 and D2, respectively (Figure 1). The distance from Pct to the pulmonary nodule was measured line by line, as CT does not support the measurement of curve length. For accuracy of the measurement, more line segments were selected to ensure that each line was close to the chest wall. The average of all values was obtained from the measurements by three reviewers. They were part of the study team. (III) The positions of Pd1 and Pd2 were determined by counting the ribs on the body surface. The body surface was marked according to D1 and D2, respectively. The intersection of the two lines was routinely identified as the puncture point (Psf) (Figure 2A). Where two lines did not intersect, the CT measurements and intercostal space counts on the body surface were repeated. (IV) In obese patients and women with a wide range of breast activity, If the anterior midline is used as a reference, when the patient changes from the supine position to the lateral position, the positioning point will change due to the swing of the breast. A line (Lsf) on the body surface was drawn, and the distance from this line to the posterior median line noted as D1 (Figure 2B). The method was due to difficulties associated with calculating the intercostal space on the body surface.
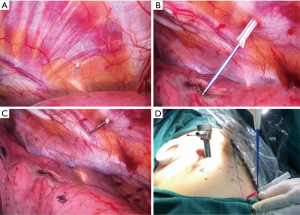
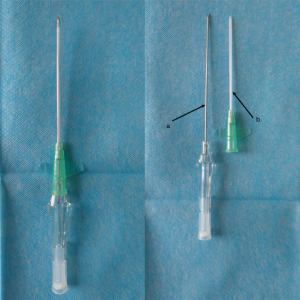
Intraoperative procedures: (I) the patient took a lateral position after the administration of general anesthesia. (II) With lungs deflated, Psf was verified by counting the ribs in the thorax with the aid of a thoracoscope. The puncture system was inserted into the chest wall with the tip extended near the parietal pleura, under direct vision of the thoracoscope (Figure 3). (III) Subsequently, the lung was fully inflated before deployment of the puncture needle (Becton Dickson Infusion Therapy Systems Inc., Sandy, Utah 84070, USA; Figure 4) into the lung parenchyma, approximately 0.3 cm distal to the visceral pleura. Electrocoagulation was performed, marking the visceral pleura accordingly (Figure 3). Pulmonary nodules (Figure 5) were located by observing the electric coagulating point. Resections were performed with a boundary of about 2 cm around the location point. The depths of the resection were mainly based on the depth of the lung nodules.

The entire IBMI location method is divided into two major steps: preoperative procedures and intraoperative procedures (Figure 6). The basis for accurate positioning was as follows: (I) CT scans were performed after full inhalation, with the breath continuously held. The intraoperative puncture was performed when the lung was completely inflated, eliminating the effect of tidal volume on the localization of pulmonary nodules. (II) Lsf and D1 were measured before the operation and marked on the body, without error. The possibility of inaccurate measurements was isolated to the reduced intercostal level. These results were overcome by confirming and correcting its location by thoracoscopy during the operation. Psf could not be determined in obese patients or those with a narrow intercostal space. The only measurements determined in these patients were Psf and D1 before the operation. However, through the intraoperative endoscopic confirmation of the intercostal level, all pulmonary nodules were successfully located. Moreover, all Psfs were located on the Lsf. (III) Thoracic adhesion had little effect on localization as it was initially separated, then located, if found during the operation.
Statistical analysis
Data analysis was conducted with standard statistical software (IBM SPSS Statistics, version 22.0, released 2013; IBM Corp, Armonk, NY, USA). The descriptive results are presented as mean ± standard deviation with range for continuous variables unless otherwise indicated. Categorical variables are expressed as percentages or numbers. Comparisons between two groups were made using the t-test or chi-square test.
Results
Patient and GGN characteristics
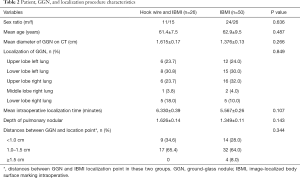
In this study, 76 patients underwent intraoperative localization between January 2018 and March 2019. No differences in distances were observed between the GGN and the hook wire or the GGN and electric coagulation burning point (Table 1) across the respective patient groups. In the IBMI + hook wire group, hook wire dislodgement was observed in one case (3.8%). The results were potentially attributed to an accidental penetration of the wire into the horizontal fissure.

Full table
The remaining 50 patients were treated with the IBMI method only. The patients characteristics such as gender, age and the data of GGN are summarized in Table 2. The statistical results (Table 2) displayed no difference between the hook wire + IBMI and the group of IBMI only in gender ratio, mean age, mean diameter of GGN on CT, localization of GGN, mean intraoperative localization time and depth of pulmonary nodules. The mean diameter of the GGNs was 1.2±0.6 (range, 0.8–2.6) cm, and the mean depth of pulmonary nodules was 1.4±0.9 (range, 0.3–2.9) cm. There were 22 nodules located in the right upper lobe, 3 in the right middle lobe, 10 in the right lower lobe, 18 in the left upper lobe, and 23 in the left lower lobe.
Full table
IBMI intraoperative localization
Data for the distances between GGNs and localization points indicated no significant differences between the two groups. Intraoperative localizations were successfully performed in 72 pulmonary nodules within a mean duration of 5.3±1.8 (range, 2.0–9.6) minutes. The GGNs in 4 cases were found to have a significant deviation (>1.5 cm) from the positioning point. In 49 cases, the distance ranged from 1.1 to 1.5 cm. In the other 23 cases, the location point was located alongside the nodule, less than 1.0 cm from the GGN (Table 1). In patients with a hook wire and IBMI localizations, no significant differences between hook wire and IBMI location points (Table 2) were observed.
Complications
One case (3.8%) of the pleural reaction was noted in patients who underwent hook wire localization. The IBMI localization procedure was well tolerated in all patients, without any significant complications or procedure-related mortality. Four cases of active bleeding (5.2%), 1 case of subpleural hemorrhage (1.3%), and no other intra- or postoperative complications were observed.
Pathological results
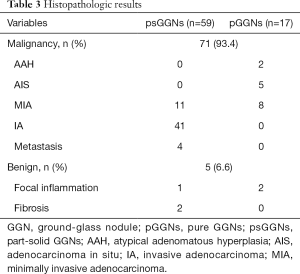
The final pathological diagnoses of all 76 lesions are reported in Table 3. All operative margins were histologically free of tumors. Forty-one psGGNs were invasive adenocarcinoma (IA), 11 psGGNs, and eight pGGNs were minimally invasive adenocarcinoma (MIA), and five pGGNs were adenocarcinoma in situ (AIS). Two pGGNs were atypical adenomatous hyperplasia (AAH). Four psGGNs were metastatic tumors (two from breast cancer and two from colorectal cancer). Three psGGNs and two pGGNs were benign lesions.
Full table
Discussion
Preoperative localization of ground-glass opacity (GGO) or a small nodule helps guide resection through VATS and reduces the incidence of conversion rate to an open procedure (5). The success rate of the micro-coil, hook wire, and the fiducial marker was reported to be 93–98.4%, with complications occurring in 3–10% of patients, including migration of the coil, air embolism, and hemothorax (18). The hook wire technique presents several limitations, among which the most important is the need for strict coordination between the CT section and the surgery room, enabling immediate patient transport to the operating room after the procedure. Alternatively, the procedures could be performed in the same operating room, if feasible. Giunta et al. reported successful CT-guided placements of a self-expanding tract sealant device (BioSentry) in all three patients before surgery without complications (19). The self-expanding tract sealant device was created for the reduction of pneumothorax and chest tube placement rates after percutaneous lung biopsy. Imperatori et al. reported 1 of 27 (4%) hydrogel plug marking procedures caused a clinically relevant pneumothorax that required chest tube drainage. The plug minimized clinically relevant pneumothoraxes and allowed flexible surgical schedules. However, a chest radiograph should be obtained 2 h later to check for the occurrence of pneumothorax. Another significant limitation is case selection, where eighty-six percent of nodules were solid, and only 4 ground-glass opacities (20). Other factors during localization and surgery, such as discomfort, tension, and hemorrhage (10-13), may still exist. Our findings demonstrate the IBMI localization approach with a significantly high success rate (94.7%). This method can locate GGNs with a minimum diameter of 0.8 cm without case selection. The GGNs were found to have a significant deviation (>1.5 cm) from the positioning point in four cases. In one case, we were unable to puncture vertically due to the occlusion of the scapula. Therefore, the other patients with scapular occlusion underwent pre-placement of electric hooks. During the operation, the puncture point was determined under the direct view of a thoracoscope, with the electric hook placed on the puncture point in advance, which could be directly located by electrocoagulation after lung expansion, avoiding puncture. One female patient had a measurement error due to the extensive range of breast movement. As a result, other female patients with excessive breast mobility underwent posterior midline marking. The other two patients with positioning failure may have failed to inhale fully during the preoperative CT scan, resulting in inconsistencies between preoperative and intraoperative positions. The IBMI localization approach could be available in most institutes with low additional equipment requirements. The IBMI localization approach is cost-effective, as it only requires a puncture needle. As the procedure was performed under general anesthesia, patients experienced minimal discomfort and complications. Furthermore, this method is deemed as a safe alternative, with no additional radiation exposure to either the surgeon or the patient. This technology has been adopted in multiple centers, with positive feedback. It is a procedure that can be learned and applied rapidly without requiring extensive practical experience. In our study, the mean intraoperative localization time was 5.3±1.8 (range, 2.0 to 9.6) minutes, highlighting its efficiency, as all non-invasive procedures for measurement and marking were performed before surgery.
Five factors significantly influenced the success rate: (I) identifying the puncture level by selecting the first rib with HRCT. The technique more accurately identified the intercostal plane, as the first rib displayed the smallest range of movement. (II) The ribs must not block the puncture path. A puncture direction perpendicular to the skin was critical in ensuring that the needle was in the right direction. (III) The choice of the puncture level was crucial. Difficulties in determining the intercostal level in some patients were overcome by bending to the opposite side with the arm lifted. Moreover, the selection of the puncture level was determined again by thoracoscopy during the operation. (IV) Obtaining the mean value of multiple reviewer’s measurements significantly reduced measurement errors. (V) The CT scan was completed during the maximum inspiratory phase to ensure that the pulmonary nodules were as consistent as possible with the preoperative position after full lung expansion during surgery.
This positioning method also has some shortcomings: The main weakness of this technique was the lack of any means to verify that the nodule has been marked successfully before cutting the lung tissue. When the first localization failed, supplemental wedge resection, or salvage segmentectomy, based on the location of the lung segment where the nodule is located, would be performed. If the nodule is missed, lobectomy should be considered. Dependency on external markers is another major weakness of this technique. The dependency would create a significant problem with populations where the average BMI is commonly higher, as superficial tissue movement based on position could negatively impact accuracy. This issue is also anticipated to be observed in large-breasted women on an ongoing basis. We obtained preoperative CTs with the patient in the lateral position, maybe of value. However, further comparative studies with a prospective randomized design are needed to define the optimal position for lung nodule/GGO localization. Some anatomical locations would be a limitation for the procedure, including apical and diaphragmatic localization, along with sites near the great vessels. Surgeons should consider other localization techniques for such nodules.
Intraoperative localization can avoid patient transport, reduce patient discomfort, and might be routinely utilized in the future. Due to the limitations on facilities or operation time in different institutes, the application of most intraoperative localization methods has been limited. The IBMI localization approach is a non-invasive intraoperative localization method that has no particular requirement for special equipment. Given its high success and low complication rate, we propose that the IBMI localization approach be the preferred choice for solid tumors and GGNs in the future.
Acknowledgments
Funding: This study was supported by the Huzhou Science and Technology Bureau (2018-GY39) and Zhejiang Medical and Health Science and Technology Plan Project (2020PY025).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-19-947). The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved and supervised by the Medical Ethics Committee of the First People’s Hospital of Huzhou. Every patient signed the informed consent form voluntarily before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakao M, Yoshida J, Goto K, Ishii G, Kawase A, Aokage K, Hishida T, Nishimura M, Nagai K. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [Crossref] [PubMed]
- Huang W, Ye H, Wu Y, Xu W, Tang X, Liang Y, Zheng J, Jiang H. Hook wire localization of pulmonary pure ground-glass opacities for video-assisted thoracoscopic surgery. Thorac Cardiovasc Surg 2014;62:174-8. [PubMed]
- Chao YK, Pan KT, Wen CT, Fang HY, Hsieh MJ. Preoperative CT versus intraoperative hybrid DynaCT imaging for localization of small pulmonary nodules: a randomized controlled trial. Trials 2019;20:400. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, Spaggiari L. Radio-guided localization and resection of small or ill-defined pulmonary lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, Hsieh MS, Ko WC, Chen JY, Hsu HH, Chang YC, Chen JS. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Wu TT, Chang YC, Lee JM, Hung MH. Anaphylactic reaction to patent blue V used in preoperative computed tomography-guided dye localization of small lung nodules. J Formos Med Assoc 2016;115:288-9. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, Kwack MS, Kim SW, Kwon OK, Jang HS. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, Clifton JC, English J, Leo J, Lam S. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Seo JM, Lee HY, Kim HK, Choi YS, Kim J, Shim YM, Lee KS. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, Hu H, Luo X, Zhang Y, Chen H. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, Nicoletti R, Del Maschio A, Zannini P. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Miyoshi K, Toyooka S, Gobara H, Oto T, Mimura H, Sano Y, Kanazawa S, Date H. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36:378-82. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Gow KW, Saad DF, Koontz C, Wulkan ML. Minimally invasive thoracoscopic ultrasound for localization of pulmonary nodules in children. J Pediatr Surg 2008;43:2315-22. [Crossref] [PubMed]
- Keating JJ, Kennedy GT, Singhal S. Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2015;149:e51-3. [Crossref] [PubMed]
- Carcoforo P, Feo C, Sortini D, Pozza E, Carrella G, Sortini A. Localization of pulmonary nodules. Chest 2004;125:796-7. [Crossref] [PubMed]
- Hwang S, Kim TG, Song YG. Comparison of hook wire versus coil localization for video-assisted thoracoscopic surgery. Thorac Cancer 2018;9:384-9. [Crossref] [PubMed]
- Giunta D, Daddi N, Dolci G, Campisi A, Congiu S, Buia F, Bagni A, Dell'Amore A. A new image-guided technique for intraoperative localization of lung small solid nodules or ground-glass opacities with a self-expanding tract sealant device: a preliminary experience. Interact Cardiovasc Thorac Surg 2019;28:23-8. [Crossref] [PubMed]
- Imperatori A, Fontana F, Dominioni L, Piacentino F, Macchi E, Castiglioni M, Desio M, Cattoni M, Nardecchia E, Rotolo N. Video-assisted thoracoscopic resection of lung nodules localized with a hydrogel plug. Interact Cardiovasc Thorac Surg 2019;29:137-43. [Crossref] [PubMed]


