Age related reduction of T1rho and T2 magnetic resonance relaxation times of lumbar intervertebral disc
Introduction
Intervertebral disc degeneration is a process that begins early in life and is the consequence of a variety of genetic, mechanical, traumatic and nutritional factors, as well as normal aging (1). Early signs of disc degeneration are manifested by biochemical changes, including a loss of proteoglycans, a loss of osmotic pressure and hydration (1). In the later stages of disc degeneration, morphological changes occur, including a loss of disc height, disc herniation, annular tears and radial bulging (2). Magnetic resonance imaging (MRI) is commonly used for assessment of symptomatic disc degeneration. On T2-weighted MR images, disc degeneration is seen as a reduction in signal of the nucleus pulposus and inner fibres of the annulus. With more severe disc degeneration, disc height decreases. Quantitative disc MR T2 and T1rho relaxation time measurements that reflect the intrinsic material properties of disc tissues are currently being explored (3-10). T2 relaxation time measurement has been reported to be sensitive to changes in collagen and water content in the intervertebral discs, and T2 relaxation time decreases with disc degeneration (3-6). T1rho relaxation measurement, which probes the interaction between water molecules and their macromolecular environment, is suggested to have the potential to identify early biochemical changes in the intervertebral disc. In cadaveric human discs it was shown that in the nucleus pulposus T1rho strongly correlates with proteoglycan content (7). In vivo studies have demonstrated differences in mean T1rho values between the nucleus and the annulus and have shown a correlation between T1rho values and degenerative grades (8,9). While T1rho has shown a distinct advantage over T2 in liver fibrosis evaluation (10-15), whether and how T1rho specifically offer better evaluation of disc degeneration compared with T2 remains poorly defined (16). The purpose of the current in vivo 3.0-T MRI study is to determine the relative performance of T1rho and T2 relaxation times in their assessment of disc degeneration associated with aging.
Materials and methods
Subjects
Fifty-two subjects were recruited into this study: 12 subjects without low back pain (9 males and 3 females; mean age: 32.1 years, age range: 23-42 years), and 40 subjects who had low back pain (17 males and 23 females; mean age: 54.1 years, age range: 28-76 years). All subjects were confirmed to have no other spine diseases except disc degeneration. The study was approved by the local human research ethics committee. Written informed consent was obtained from all subjects.
Magnetic resonance imaging (MRI)
To remove the potential confounding role of diurnal disc hydration changes, all subjects underwent imaging in the morning. MRI acquisition was performed on a 3-T clinical system (Achieva, Philips Healthcare, Best, The Netherlands). A 12-channel receive-only spine coil was used as the signal receiver to cover the lumbar spine, and the built-in body coil was used as the signal transmitter. Volume shimming was employed to minimise B0 heterogeneity.
For T1rho measurement, a rotary echo spin-lock pulse was implemented in a 3D balanced fast field echo (b-FFE) sequence (17). Spin-lock frequency was set as 500 Hz and the spin-lock times (TSLs) of 1, 10, 20, 30, 40 and 50 ms were used for acquisition and T1rho mapping. Segmented phase alternating b-FFE readout with centric phase encoding order was used for acquisition. T1rho-weighted images were acquired during the transient status towards the steady state but with T1rho-weighted magnetisation maintained (18). A rotary echo spin-lock pulse was applied once for every segment length of 80 readouts. A dummy delay time (TD) of 6,000 ms was inserted after each segment acquisition to fully restore the equilibrium magnetisation before the next T1rho preparation. TE and TR for b-FFE acquisition were 2.3 and 4.6 ms respectively. The field-of-view (FOV) was 200 mm and the voxel size was 1.0 mm × 1.0 mm. Seven sagittal slices were acquired with the slice thickness of 4 mm. The flip angle was 40° and the number of signal averages (NSA) was one. A sensitivity-encoding (SENSE) factor of 2 was applied for parallel imaging to reduce the phase encoding steps. A multi-echo turbo spin echo (TSE) pulse sequence was used for T2 mapping. Seven sagittal TSE images were acquired at identical locations as T1rho images. TSE imaging parameters included: FOV =200 mm, pixel =1.0 mm × 1.0 mm, slice thickness =4 mm, echo train length (ETL) =7, TEs =16, 32, 48, 64, 80, 96 and 112 ms, TR =2,300 ms, NSA =1 and SENSE factor =2.
Image analysis
T1rho and T2 maps were computed on a pixel-by-pixel basis using a mono-exponential decay model with a home-made Matlab program (Mathworks, Natick, MA, USA):
M(TSL) = M0*exp(-TSL/T1rho) and
M(TE) = M0*exp(-TE/T2)
where M0 and M(TSL) denote the equilibrium magnetisation and T1rho-prepared magnetisation with the spin-lock time of TSL, respectively. M(TE) denotes the magnetisation acquired with the echo time TE.
These two mono-exponential equations were linearised by logarithm. T1rho and T2 maps were generated by fitting each pixel’s intensity as a function of TSL and TE using a non-negative least-square fitting algorithm, respectively. T1rho and T2 were calculated as the inverse of the slope of the corresponding straight-line fit (19).
Five intervertebral discs (L1/L2-L5/S1) per subject were examined, with four discs excluded because of previous vertebral fusion operation, leading to 256 discs in total for analysis. Subjects with or without low back pain were analysed together. Images were analysed in the mid-sagittal section of the lumbar spine. With T2-weighted images as reference, regions of interest (ROIs) were manually drawn over the T2 map and T1rho map of the discs by a radiologist (16,20). ROIs included nucleus pulposus, anterior annulus fibrosus and posterior annulus fibrosus (Figure 1). The nucleus pulposus and inner annulus fibrosus can show nearly the same high signal on T2 weighted images and following disc degeneration inner annulus fibrosus also shows a lower signal, therefore a clear border between them cannot be defined. Values of anterior annulus fibrosus and posterior annulus fibrosus were averaged as the value for annulus fibrosus. When an apparent tear in was noted in the annulus, the abnormal signal areas were excluded in the ROIs. The ROI size for nucleus pulposus ranged 15-45 mm2, while the ROI size for annulus fibrosus (anterior + posterior) ranged from 8 to 45 mm2. Using such an approach the core part of nucleus pulposus was measured, and part of inner annulus fibrosus might have been missed.

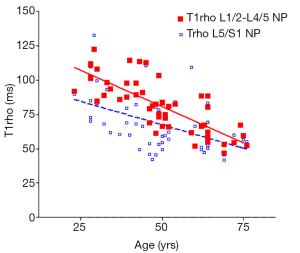
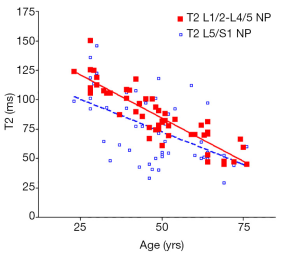
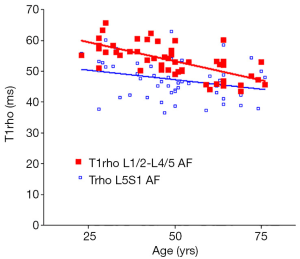
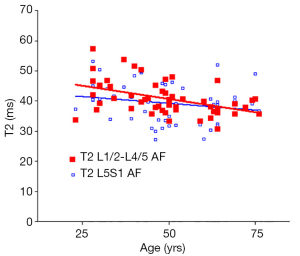
As previously reported (18,19), discs L1/2-L4/5 (four discs) and disc L5/S1 were analyzed separately. The mean value of T1rho and T2 relaxation times of L1/2-L4/5 was regarded as the value of the subject, and plotted against the age of the subjects using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA 92037, USA). Goodness of fit r2, slope, and P value tested for non-zero was obtained. The slopes of T1rho/T2 reduction over age were compared using t-test.
Results
There was a significant trend of reduction of T1rho and T2 relaxation times as the subjects’ age increased. This reduction was faster (slope steeper) in nucleus pulposus compared with that of annulus fibrosus (Figures 2,3,4,5). The T2 slope was slightly steeper for nucleus pulposus, while T1rho slope was slightly steeper for annulus fibrosus (Figures 6,7).
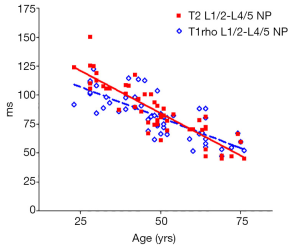
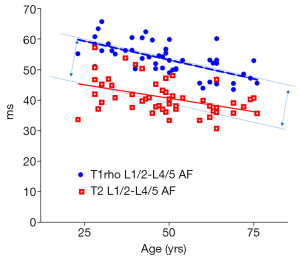
Discussion
The current study was composed of both non-symptomatic volunteers as well as patients with low back pain. We evaluated 23- to 76-year-old subjects, an age range in which a broad spectrum of disc degeneration, including early degeneration, is expected. In this study, both T1rho mapping and T2 mapping were acquired, and nucleus pulposus and annulus fibrosus were analysed separately. MR images were acquired sagittally so that all the five lumbar discs can be analysed in a single plane. Non-symptomatic volunteers and patients with low back pain were grouped together, as only a weak correlation exists between disc degeneration and clinical symptoms (21,22), and imaging findings of degenerative disc disease do not predict subsequent symptom development (23). Additionally, it cannot be certain that the back pain of our patients was disc related.
The role of specific biochemical changes in the altered MR signal intensity during disc degeneration is still not well understood. In the articular cartilage study, the loss of proteoglycan results in an increase in T1rho relaxation time (24). On the other hand, T1rho is reported to increase with sulphated-glycosaminoglycan content in degenerative discs (7). Nucleus pulposus is composed of abundant sulphated glycosaminoglycans in a loose network of type II collagen. It is a hydrated gel containing approximately 25% (dry weight) collagen and 50% (dry weight) proteoglycan (25). The proteoglycans of the nucleus osmotically exert a “swelling pressure”, which enables it to support spinal compressive loads. In comparison, nucleus pulposus is made up of coarse type I collagen fibres, and contains 67% (dry weight) collagen and a low concentration of proteoglycans (25,26). During the initial phase of disc degeneration, loss of proteoglycans and collagen type II is observed (27). Proteoglycan loss reduces the capacity to bind water and leads to a loss of hydration. Later, type I collagen fibres replace the type II collagen fibres in the annulus, thus altering the tensile properties of the tissue. The reduced water content is a contributing factor of the reduction of both T1rho and T2 relaxations with degenerated discs (7). It is known that there is a reduction of T2 in degenerated discs. As disc degeneration is associated with aging, it is not surprising that T2 reduction is observed in older subjects. Niu et al. (28) reported an over all age-related reduction of T2 value in the disc region of nucleus pulposus and inner annulus fibrosus. Wu et al. (29) demonstrated significant disc region T2 difference between young (age <45 years) and elderly group (age >45 years). However, to our knowledge, in vivo result of T1rho over aging and result concerning annulus fibrosus have not yet been reported in the literature.
There are many limitations of our study. The disc degeneration seen in this study cannot all be considered due to aging. MR images do not allow us to clearly separate nucleus pulposus and inner annulus fibrosus. The reduction of MR relaxation times over aging may not actually follow a linear mode. And the patient number is small which prevented us from more detailed subgroup analysis, such as the gender effect (30). Therefore the results described in this study should be considered as preliminary.
In conclusion, our study demonstrated significant age related reduction of T1rho and T2 magnetic resonance relaxation times both in the nucleus pulposus and the annulus fibrosus of lumbar intervertebral disc. However, the relative performances of T1rho vs. T2 were broadly similar. To explore the advantage of T1rho over T2 for disc degeneration assessment, more studies are required.
Acknowledgements
This study was partially supported by the Research Grants Council of the Hong Kong SAR (Project No: SEG_CUHK02).
Disclosure: The authors declare no conflict of interest.
References
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151-61. [PubMed]
- Modic MT, Ross JS. Lumbar degenerative disk disease. Radiology 2007;245:43-61. [PubMed]
- Marinelli NL, Haughton VM, Muñoz A, Anderson PA. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine (Phila Pa 1976) 2009;34:520-4. [PubMed]
- Kerttula L, Kurunlahti M, Jauhiainen J, Koivula A, Oikarinen J, Tervonen O. Apparent diffusion coefficients and T2 relaxation time measurements to evaluate disc degeneration. A quantitative MR study of young patients with previous vertebral fracture. Acta Radiol 2001;42:585-91. [PubMed]
- Takashima H, Takebayashi T, Yoshimoto M, Terashima Y, Tsuda H, Ida K, Yamashita T. Correlation between T2 relaxation time and intervertebral disk degeneration. Skeletal Radiol 2012;41:163-7. [PubMed]
- Chiu EJ, Newitt DC, Segal MR, Hu SS, Lotz JC, Majumdar S. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine (Phila Pa 1976) 2001;26:E437-44. [PubMed]
- Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 2006;31:1253-7. [PubMed]
- Blumenkrantz G, Li X, Han ET, Newitt DC, Crane JC, Link TM, Majumdar S. A feasibility study of in vivo T1rho imaging of the intervertebral disc. Magn Reson Imaging 2006;24:1001-7. [PubMed]
- Auerbach JD, Johannessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliott DM. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J 2006;15 Suppl 3:S338-44. [PubMed]
- Wang YX, Yuan J, Chu ES, Go MY, Huang H, Ahuja AT, Sung JJ, Yu J. T1rho MR imaging is sensitive to evaluate liver fibrosis: an experimental study in a rat biliary duct ligation model. Radiology 2011;259:712-9. [PubMed]
- Zhao F, Wang YX, Yuan J, Deng M, Wong HL, Chu ES, Go MY, Teng GJ, Ahuja AT, Yu J. MR. T1ρ as an imaging biomarker for monitoring liver injury progression and regression: an experimental study in rats with carbon tetrachloride intoxication. Eur Radiol 2012;22:1709-16. [PubMed]
- Wang YX, Zhao F, Wong VW, Yuan J, Kwong KM, Chan HLY. Liver MR T1rho measurement in liver cirrhosis patients: a preliminary study with a 2D fast field echo sequence at 3T. Proc Intl Soc Mag Reson Med 2012;20:
- Allkemper T, Sagmeister F, Cicinnati V, Beckebaum S, Kooijman H, Kanthak C, Stehling C, Heindel W. Evaluation of fibrotic liver disease with whole-liver T1ρ MR imaging: a feasibility study at 1.5 T. Radiology 2014;271:408-15. [PubMed]
- Rauscher I, Eiber M, Ganter C, Martirosian P, Safi W, Umgelter A, Rummeny EJ, Holzapfel K. Evaluation of T1ρ as a potential MR biomarker for liver cirrhosis: comparison of healthy control subjects and patients with liver cirrhosis. Eur J Radiol 2014;83:900-4. [PubMed]
- Wang YX, Yuan J. Evaluation of liver fibrosis with T1ρ MR imaging. Quant Imaging Med Surg 2014;4:152-5. [PubMed]
- Wang YX, Zhao F, Griffith JF, Mok GS, Leung JC, Ahuja AT, Yuan J. T1rho and T2 relaxation times for lumbar disc degeneration: an in vivo comparative study at 3.0-Tesla MRI. Eur Radiol 2013;23:228-34. [PubMed]
- Charagundla SR, Borthakur A, Leigh JS, Reddy R. Artifacts in T(1rho)-weighted imaging: correction with a self-compensating spin-locking pulse. J Magn Reson 2003;162:113-21. [PubMed]
- de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, Koes BW, Bierma-Zeinstra SM. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 2010;35:531-6. [PubMed]
- Wang YX, Griffith JF, Zeng XJ, Deng M, Kwok AW, Leung JC, Ahuja AT, Kwok T, Leung PC. Prevalence and sex difference of lumbar disc space narrowing in elderly chinese men and women: osteoporotic fractures in men (Hong Kong) and osteoporotic fractures in women (Hong Kong) studies. Arthritis Rheum 2013;65:1004-10. [PubMed]
- Wang YX, Zhao F, Yuan J, Mok GS, Ahuja AT, Griffith JF. Accelerated T1rho relaxation quantification in intervertebral disc using limited spin-lock times. Quant Imaging Med Surg 2013;3:54-8. [PubMed]
- Andersson GB. Epidemiology of low back pain. Acta Orthop Scand Suppl 1998;281:28-31. [PubMed]
- Peterson CK, Bolton JE, Wood AR. A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine (Phila Pa 1976) 2000;25:218-23. [PubMed]
- Borenstein DG, O’Mara JW Jr, Boden SD, Lauerman WC, Jacobson A, Platenberg C, Schellinger D, Wiesel SW. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: a seven-year follow-up study. J Bone Joint Surg Am 2001;83-A:1306-11. [PubMed]
- Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, Reddy R. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 2001;46:419-23. [PubMed]
- Cassinelli EH, Hall RA, Kang JD. Biochemistry of intervertebral disc degeneration and the potential for gene therapy applications. Spine J 2001;1:205-14. [PubMed]
- Weidenbaum M, Foster RJ, Best BA, Saed-Nejad F, Nickoloff E, Newhouse J, Ratcliffe A, Mow VC. Correlating magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res 1992;10:552-61. [PubMed]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 1996;98:996-1003. [PubMed]
- Niu G, Yang J, Wang R, Dang S, Wu EX, Guo Y. MR imaging assessment of lumbar intervertebral disk degeneration and age-related changes: apparent diffusion coefficient versus T2 quantitation. AJNR Am J Neuroradiol 2011;32:1617-23. [PubMed]
- Wu N, Liu H, Chen J, Zhao L, Zuo W, Ming Y, Liu S, Liu J, Su X, Gao B, Tang Z, Qiu G, Ma G, Wu Z. Comparison of apparent diffusion coefficient and T2 relaxation time variation patterns in assessment of age and disc level related intervertebral disc changes. PLoS One 2013;8:e69052. [PubMed]
- Wang YX, Griffith JF. Effect of menopause on lumbar disk degeneration: potential etiology. Radiology 2010;257:318-20. [PubMed]


