
Early minimal lesions of COVID-19 pneumonia with interstitial lung abnormality: a case description
Introduction
Since December 2019, many cases of pneumonia caused by a novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] have emerged in Wuhan, China. By March 20, 2020, the number of cases confirmed with coronavirus disease 2019 (COVID-19) infection reached 266,073 globally and 11,184 cases died totally (1). Real-time polymerase chain reaction (RT-PCR) test for COVID-19 has been developed and widely used in clinics. In addition, chest CT can play an important role in detecting COVID-19 pneumonia. Hereby, we describe a confirmed case of COVID-19 pneumonia in a 66-year-old male. We present the following case in accordance with the CARE Guideline. A completed CARE guideline checklist is available at http://dx.doi.org/10.21037/qims-20-471.
Case report
A 66-year-old male presented with fever (37.8 °C), cough, nasal congestion, runny nose, sore throat and little yellow sticky sputum, came to our pulmonology department on January 24th, 2020. He had a residence history in Xiaogan (75 kilometres away from Wuhan), Hubei Province and drove to Shijiazhuang, Hebei Province, with his family (his wife, son, daughter-in-law and grandson) on January 22nd, 2020. Unenhanced computed tomography (CT) of the chest was not preformed and routine blood examination results showed both normal leukocyte count (5.13×109/L) and lymphocyte count (1.57×109/L).
After taking cefixime, oseltamivir, and “Lianhua Qingwen Granule” (a type of Chinese traditional medicine) at home, the fever resolved for 1 day. He visited the infectious disease department for further diagnosis and treatment when the body temperature slightly increased on January 26th, 2020. Physical examination showed fever with a body temperature of 37.5 °C, and the laboratory examination results indicated slightly elevated C-reactive protein (10.51 mg/L; normal range, <10 mg/L), normal leukocyte count (5.99×109/L), normal neutrophils (67.7%), normal lymphocyte count (1.35×109/L), normal blood platelet count (182.00×109/L). Tests for influenza A virus and influenza B virus by colloidal gold-labeled method were negative.
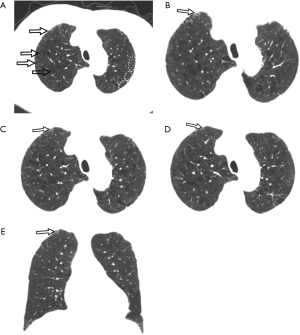
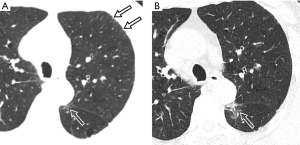
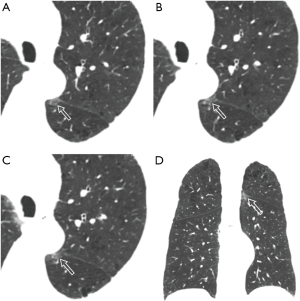
Unenhanced chest CT scan, initially performed on January 26th, depicted mild centrilobular emphysema in bilateral upper lobes with predominantly mild paraseptal emphysema, multiple regional subpleural interlobular septal thickening (Figure 1) and multiple streaky opacities bilaterally. Given minimal ground-glass opacity (GGO) was detected with chest CT between left oblique fissure and paravertebral area in the superior segment of left lower lobe (Figure 2), interstitial lung disease was considered together with the manifestation of bilateral upper lobes. Since the patient had an epidemiological history (residence in Xiaogan) with fever that self-treatment did not cure and normal blood routine examination result, he was considered as a suspected case with SARS-CoV-2 infection and real-time polymerase chain reaction (RT-PCR) test of throat swab sample was taken. The patient was sent to designated hospital for treatment immediately after the positive RT-PCR result was found. He had a smoking history of 20 years (30 cigarettes per day) but had quit smoking for 20 years. He also had a 2-year history of paroxysmal atrial fibrillation with irregular medication.
This patient was the first case with SARS-CoV-2 infection detected in the family cluster. His wife, who contacted with the patient, developed fever and cough while being isolated and was sent to another designated hospital on January 27th. She was confirmed by positive RT-PCR result 7 days after initial onset of symptoms. His son-in-law, who had visited the patient at Xiaogan on January 15th, had a positive RT-PCR result as well and was sent to the same designated hospital on February 3rd. There was no fever in the rest of the family members during quarantine and their twice RT-PCR results were both negative.
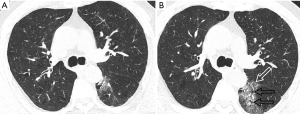
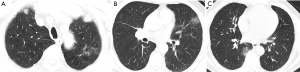
During the treatment at designated hospital, repeat chest CT scans were performed. There was rapidly expansion of GGO in the left lower lobe with reticulate interlobular septa thickening (Figure 3). In addition, chest CT at 9 days after symptom onset, depicted additional two GGOs in the left lung apex and the left inferior lingular segment (Figure 4). Another GGO in the posterior basal segment of right lower lobe was present on chest CT at 21 days. Three lesions of left lung gradually expanded within the first half of hospitalization. After 27 days of antiviral and supportive treatment, the last chest CT showed significant absorption of the three lesions in left lung while no change of the lesion in right lung. He was discharged after negative RT-PCR results consecutively tested for two times.
Discussion
COVID-19 is a pulmonary infectious disease caused by SARS-CoV-2 infection, which is commonly characterized by fever, dry cough and fatigue. Pathologic data from autopsy indicated diffuse alveolar damage with cellular fibromyxoid exudates, desquamation of pneumocytes and hyaline membrane formation (2). There can be interstitial mononuclear inflammatory infiltrates in both lungs, which consists mainly of lymphocytes.
Bernheim et al. reported 56% patients with SARS-CoV-2 infection had no lung opacities found in the chest CT performed within 2 days after symptom onset, as compared with 9% in 3–5 days and 4% in 6–12 days (3). Xu et al. noted that patients with SARS-CoV-2 infection might show negative CT performance at initial and follow-up (4). However, different radiological patterns of abnormalities could be found in subclinical patients, including unilateral (60%), multifocal (53%) and ground-glass opacification (93%). Patterns of lesions had quick developments to bilateral (90%), diffuse (52%) within a week after symptom onset (5).
In this patient, unilateral, unifocal GGO in the superior segment of left lower lobe was detected in the initial chest CT performed 3 days after symptom onset (Figure 5). Later another GGO emerged in the posterior basal segment of right lower lobe, leading to both lung involvement finally. And GGOs had a development to multiple lesions with expansion, consistent with SARS-CoV-2 infection. The minimal GGO in the superior segment of left lower lobe should be COVID-19 pneumonia at early stage. However, it did not attract more attention at the first time and was misdiagnosed as interstitial lung disease. Therefore, radiologists should pay more attention to such imaging feature and avoid missed diagnosis.
The following points were summarized deliberately after this missed diagnosis. Firstly, at early stage of COVID-19 pneumonia (0–3 days after onset of symptom), although there were symptoms and laboratory findings, pathologic changes might be still restricted in the alveolar wall of the terminal respiratory unit without exudation into alveolar space. Abnormalities at this stage could be only detected by histological examination, but not high-resolution CT. Therefore, chest CT may depict normal presentation or minimal lesions. Tian et al. (6) reported histopathology of the resected pulmonary segments due to adenocarcinoma, being retrospectively found to have had COVID-19 pneumonia at the time of surgery, exhibited edema, proteinaceous exudate with globules, focal hyperplasia of pneumocytes with patchy inflammatory cellular infiltration, and multinucleated giant cells, and without prominent hyaline membranes.
Secondly, focal regions of low attenuation surrounded by normal lung representing centrilobular emphysema, and low attenuation adjacent to visceral pleura representing paraseptal emphysema, which could appear simultaneously in the cohort with extensive history of smoking (7), resulting in heterogeneous attenuation of bilateral upper lobes in CT images (8). Previous studies revealed positive association between paraseptal emphysema and interstitial lung abnormalities (9). The coexistence of these anomalies leads to regional low attenuation, normal lung attenuation, and high attenuation appear alternately at subpleural areas. Therefore, the GGO with high attenuation in left lower lobe at the same slice is likely misdiagnosed of interstitial lung abnormalities.
Thirdly, ground-glass opacity with periphery distribution was not the only typical feature of COVID-19 pneumonia in CT images. Lesion number and bilateral lung involvement were also crucial references for recognition. Unifocal GGO detected at early course of disease is difficult to differentiate from other viral pneumonias.
Radiologists should pay more attention to differentiate a subpleural GGO with mild interstitial abnormalities. Clinical suspicion needs to be weighed heavily especially when there is a background of interstitial lung disease. This might be even more important when RT-PCR test is not widely available in some places and may need a long time for obtaining result.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-471). The authors have no conflicts of interest to declare.
Guideline Checklist: The authors have completed the CARE guideline checklist. Available at http://dx.doi.org/10.21037/qims-20-471.
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19): situation report 61. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200321-sitrep-61-covid-19.pdf?sfvrsn=f201f85c_2, Published 21 March 2020.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-422. [Crossref] [PubMed]
- Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M, Chest CT. Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020. [Crossref] [PubMed]
- Xu R, Du M, Li L, Zhen Z, Wang H, Hu X. CT imaging of one extended family cluster of corona virus disease 2019 (COVID-19) including adolescent patients and “silent infection”. Quant Imaging Med Surg 2020;10:800-4. [Crossref] [PubMed]
- Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425-34. [Crossref] [PubMed]
- Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020. [Crossref] [PubMed]
- Smith BM, Austin JH, Newell JD Jr, D'Souza BM, Rozenshtein A, Hoffman EA, Ahmed F, Barr RG. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med 2014;127:94.e7-23. [Crossref] [PubMed]
- Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D'Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011;364:897-906. [Crossref] [PubMed]
- Araki T, Nishino M, Zazueta OE, Gao W, Dupuis J, Okajima Y, Latourelle JC, Rosas IO, Murakami T, O'Connor GT, Washko GR, Hunninghake GM, Hatabu H. Paraseptal emphysema: Prevalence and distribution on CT and association with interstitial lung abnormalities. Eur J Radiol 2015;84:1413-8. [Crossref] [PubMed]


