Assessing a recent South Korean cohort study of cancer risk following diagnostic radiation exposure at younger ages
Introduction
We welcome the recent South Korean cohort study by Hong et al. (1), which estimated cancer risks following low-dose diagnostic radiation exposure before age 20. This study captured 12,068,821 patients from a nationally representative sample of South Korean residents. The study is larger than previous paediatric CT cohort studies (2-7), exceeding the number of CT exposed individuals (n=1,179,021) of all previous studies combined; though this advantage is offset by relatively short follow-up time. Including all forms of diagnostic radiation (not just CT scans), this study had a total of 1,275,829 individuals exposed to at least one low-dose medical radiation procedure. This important study of paediatric imaging is the first from South Korea, and the second from Asia, following the work of Huang et al. (4). Our commentary compares and contrasts this new study with earlier studies, highlighting advantages, potential limitations and outstanding questions.
Cancer risks and comparison with previous studies
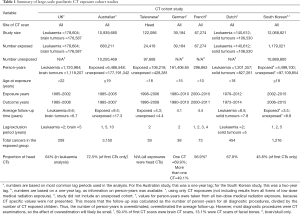
A summary of large-scale paediatric CT cohort studies is provided in Table 1. Compared to previous studies, the South Korean study had a lower proportion of head scans and a relatively short follow-up duration for the exposed group. The South Korean cohort study confirms many of the findings from earlier cohorts. For example, several studies found an increased risk of any cancer after CT exposure when compared to an unexposed population (3,5,7). However, Hong et al. was the first of the large-scale cohort studies to find a positive association between paediatric CT exposure and breast cancer risk (IRR =2.53; 95% CI, 1.44–4.43), after Australian and Dutch studies did not observe a significant association (3,7). The breast cancer results in Hong et al. are surprising, given the young age of the cohort and the small number of breast cancer cases. There were 13 breast cancer cases contributing to the analysis in the South Korean study, 145 in the Australian study, and 38 in the Dutch study (using 2-, 1-, and 5-year lags respectively).
Full table
Hong et al. also reported high incidence rate ratios (IRRs) for respiratory cancers after a chest CT (IRR =5.68; 95% CI, 2.93–11.01) and mouth and pharynx cancers after spine or neck CT (IRR =6.46; 95% CI, 3.45–12.11). However, caution is warranted as there were small numbers of cases, with only 9 respiratory cancers and 10 mouth and pharynx cancers among exposed children.
Cancer rates observed in the UK, Australian, and South Korean cohorts
Hong et al. reported that their cancer incidence was lower than that of the Australian and the UK CT studies, and suggested that “the cancer diagnoses in this study were accurate.” However, the observed difference in incidence could also reflect differences in cohort ages and follow-up times. From the numbers of CT exposed and cancer diagnoses presented in Hong et al., we calculate that 0.11% of CT exposed children were diagnosed with cancer in the South Korean cohort
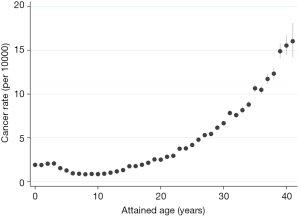
However, comparing the number of cases and number of CT exposed people is not useful when the average follow-up time and lag periods have not been accounted for; in fact, it is misleading. It is possible to compare cancer rates from the South Korean and Australian study using a one-year lag. Extracting data from Table 5 in Hong et al. we observe a cancer incidence of 3.3 per 10,000 person-years among those exposed to low-dose radiation, with an overall cancer incidence of 2.2 per 10,000 person-years across the cohort. In comparison, the Australian study observed an incidence rate of 4.9 per 10,000 person-years for the CT exposed when a one-year lag was applied (data from Table 3 in Mathews et al.), and a rate of 3.3 per 10,000 person-years for the whole cohort. There is still a discrepancy between the Australian and South Korean study, with the incidence being 1.5 times higher in the Australian study. However, this difference may be explained by the age of the cohort. The average age at first exposure was far younger in the South Korean study, with 47.2% of first diagnostic exposure occurring before age 10, compared with 21.7% in the Australian study (Table 2). Furthermore, the average length of follow-up in the South Korean is approximately half of that in the Australian study (refer to Table 1). Taken together, this means that the age distributions of the two studies were vastly different, with the South Korean study comprising a far younger cohort by the end of follow-up. In the Australian cohort, we observe an exponential increase in cancer incidence after age 20 (Figure 1). If the attained age distribution in the Australian study mimicked that of the South Korean, we would expect a lower cancer rate, more closely resembling the cancer rate observed in Hong et al.

Full table
Similarly, we can compare leukaemia and brain cancer rates among the radiation exposed children in the UK and South Korean study. For brain cancer, the UK study did observe a far higher incidence rate than the South Korean study, with an estimated 11.4 brain cancer cases per 100,000 person-years
Strengths and limitations of the South Korean CT study
The size of the cohort, with a large number of CT exposures, was an asset. Childhood cancer is rare, and the excess risk expected from low-dose radiation is rather small. Studies therefore require very large sample sizes to provide sufficient statistical power to detect any radiation effect. However, the large size of the South Korean cohort is offset by the short follow-up time, as children entered at the beginning of 2006 and exited, at latest, in December 2015. This gives a maximum follow-up of just under 10 years. For the exposed cohort, follow-up was even shorter, as entry into the exposed group was lagged, usually by two years. Additional follow-up will increase the reliability and utility of results from this cohort.
Readers should not assume the “statistically significant” relative risks seen for many cancers provide evidence of causation. Narrow confidence intervals and convincing point estimates may lead the reader to be more trusting of the observed effect than is warranted. A large sample size does not negate the effect of bias due to confounding; nor does it overcome the limitations of short follow-up time.
Bias due to confounding by indication or reverse causation
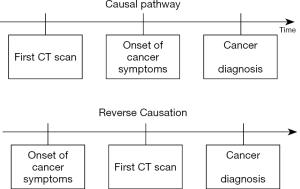
A common issue with low-dose diagnostic radiation studies is the potential for indication bias and reverse causation. Though the two terms are sometimes used interchangeably, we distinguish between them in this article. Reverse causation occurs when symptoms of undiagnosed cancer prompt the CT examination. That is, the outcome (cancer) prompted the exposure (diagnostic radiation). This has been illustrated in Figure 2.
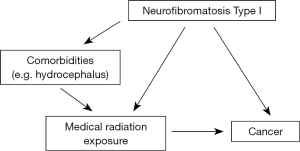
Confounding by indication, otherwise known as indication bias, occurs when the child has any cancer-predisposing conditions that requires increased exposure to medical radiation (Figure 3). An example of this is Neurofibromatosis type I, which is associated with increased risk of cancer. Children with this condition may be exposed to more diagnostic radiation than the general population, to diagnose or manage various comorbidities (e.g., hydrocephalus). An observed excess cancer risk in the exposed may be due to exposure, however it could also be attributed to the underlying pre-cancerous condition. Thus, these cancer pre-disposing conditions may confound the association between medical radiation and cancer.
Distinguishing between reverse causation and confounding by indication is important, as the biases they introduce, and the methods for handling these biases, differ. For reverse causation, one would expect a maximum increase in the excess risk in the early years of follow-up, as the pre-existing cancers are formally diagnosed. This excess risk will drop off rapidly with time, ostensibly leaving the causal association between diagnostic radiation exposure and cancer. Given that the study by Hong et al. did not exclude CT scans ordered due to suspicion of cancer, we expect there would be an element of reverse causation. Like many other medical radiation studies, Hong et al. used lag periods to address this issue. Here, children were not considered “exposed” until a period of time had elapsed after the exposure. After a “sufficient” lag, we expect that reverse causation would not meaningfully bias the estimates. Compared to the unexposed, Hong et al. observed elevated cancer risks among those exposed to any low dose diagnostic radiation with a five-year lag (IRR =1.48; 95% CI, 1.35–1.63). That the IRR remained elevated five years after exposure suggests that reverse causation could not entirely explain the results of the South Korean study.
The effects of reverse causation in the Hong et al. study were most apparent in the analysis by number of CTs, with varying lag (eTable 2 in Hong et al.). With increasing number of CT exposures, a larger attenuation in IRR with increasing lag was observed. For one CT exposure (compared to none) the IRR did not decrease substantially with increasing lag, changing from 1.49 (95% CI, 1.41–1.57) with a one-year lag, to 1.39 (95% CI, 1.24–1.55) with a five-year lag. For two CT exposures, the decrease is greater, dropping from 2.62 (95% CI, 2.31–2.98) to 1.44 (95% CI, 1.12–1.84) across the same lag periods. For ≥3 CTs the IRR decreases from 9.05 (95% CI, 7.84–10.46) with a one-year lag to 2.90 (95% CI, 2.19–3.83) with a five-year lag. The greater attenuation of IRR with increasing lag for ≥3 CT examinations is probably due to reverse causation, as children with symptoms of cancer may be repeatedly scanned within a short period of time before receiving an official diagnosis. The high IRR for three or more CT scans using a one- or five-year lag was not seen in the Australian study. The Australian study included early CT scans from 1985 to 2005, while the South Korean study included exposures from 2002 to 2013. The doses for paediatric scans have declined substantially since the 1980s, making the higher IRR in the Hong et al. study seem counterintuitive. However, the Australian study has a far higher proportion of head scans, with 72.5% of scans targeting the head, compared with 45.8% of scans in the South Korean study (Table 1). Head scans have a relatively low effective dose, compared with sites such as the abdomen. Therefore, it may still be consistent to see a greater IRR in the South Korean study, despite the years of exposure. This idea is supported by the greater IRR observed for all cancers in the South Korean study after an abdominal, chest, or spine/neck CT scan, compared with the IRR for all cancers after head CT (eTable 1 in Hong et al.).
Though the lagging approach in Hong et al. may have been sufficient to control for reverse causation, the possibility of indication bias remains. Accounting for indication bias is not always possible in large-scale studies, as indications for the diagnostic radiation exposure are usually not available. Furthermore, the effects of indication bias are not necessarily expected to diminish across follow-up time. To properly account for indication bias, medical records need to be reviewed, identifying children with high risk conditions, as in several previous studies (4,8,9). Confounding by indication may have biased the associations observed in the South Korean cohort study. Hong et al. acknowledge that the entire excess risk cannot be attributed to low-dose radiation exposure, though the extent of the bias remains unknown.
Dose-response
The South Korean cohort study did not incorporate a dose-response analysis, as scanning parameters were unavailable, precluding dose estimation. Bradford Hill’s guidelines for causality lists a biological gradient, such as a dose-response curve, as one type of evidence to support a causal interpretation (10). The authors conclude that, “the associations we found of diagnostic low-dose ionizing radiation with increased incidence of cancer in youths suggest that there is incentive to limit radiation doses to as low as reasonably achievable and to only scan when justified.” Although the South Korean study demonstrated an increased cancer risk with increasing cumulative CT scans in lieu of dose, causal inferences must be tentative without a dose-response analysis.
Exposure measurement error
As previously described, lag periods can minimise reverse causation bias when applied correctly. This generally involves delaying the entry of each CT scan by the lag duration. A CT scan will contribute to the analysis time if exit date > scan date + lag duration. The total time a CT scan contributes to the analysis time can be calculated by exit date − (scan date + lag duration).
The lag period is not always defined in such a way, and Figure 4 illustrates several scenarios where subsequent CT scans may or may not be included in the analysis, depending on the definition of the lag period. In Figure 4A,B, the first and second CT scan should contribute to the analysis at the end of the lag period, given that exit date > scan date + lag duration.
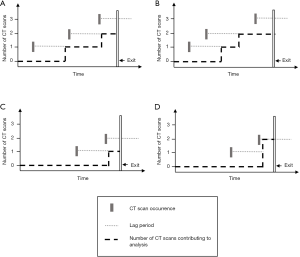
Scenario C is also a correct application of the lag period. The first scan should contribute to the analysis time as exit date > scan date + lag date. By the same logic, the second scan should not be included, as the individual has exited the study before the lag period has elapsed. Conversely, scenario D has incorrectly applied the lag period. Here, the lag period was defined as time from first scan, failing to apply the lag period to subsequent scans. Thus, the second scan has wrongly contributed to the child’s overall measured exposure level because it occurred within the lag period of the first scan, even though the child exited the study shortly after the second scan. While one can assume that the first scan was not ordered on suspicion of cancer, the same cannot be said for the second scan. Furthermore, the latency period associated with radiation-induced cancers would mean that any cancer occurring shortly after a scan is less likely to be attributable to that CT scan.
The application of the lag period in Hong et al. is somewhat ambiguous. The authors state that they “set the first exposure date as the start point of the lag period, and multiple exposures were calculated if additional exposures occurred during the lag period”, but it is not clear from this description whether their approach corresponds more closely to Figure 4C or Figure 4D.
Statistical limitations
The South Korean study made no reference to testing whether hazards were proportional in their analyses, which is an important assumption in Cox regression analyses. Furthermore, it is difficult to discern whether the reported estimates were derived from Poisson or Cox regression models. Hong et al. also did not explore interaction effects, perhaps because of the lack of data on potential interaction variables such as age and cancer predisposing conditions. In addition, although the authors state that the change in IRR with increasing lag was not “statistically significant”, they provide no description of whether or how this was tested. The phrasing “We hypothesized a lag period…” suggests that there is a true underlying lag period which can be determined. However, the authors did not formally assess the latency period (the time between exposure and diagnosis); rather, they assumed a lag period of two years (and varied it in the supplementary material). The authors also state that “statistical significance was set at P less than 0.05”, but do not make clear which results were statistically significant, and in any case actual p-values should be reported, not just whether they were above or below the arbitrary threshold of 0.05 (whilst recognising the many disadvantages of P values) (11,12). The authors’ use of floated confidence intervals in the analysis by number of CT scans is somewhat surprising, as there are differing views about the application and performance of this method (13,14). The authors offered no explanation for their decision to use of floating confidence intervals.
Despite these limitations, the South Korean study is important, as it provides additional evidence of increased cancer risk following low-dose diagnostic radiation in childhood and adolescence.
Conclusions
Results from any large cohort study, particularly when exploring the effects of CT exposure, must be interpreted with caution. The South Korean cohort study is currently the largest paediatric study estimating cancer risks following low-dose medical radiation exposure (predominately in the form of CT scans). It reported elevated risks for many cancer types following radiation exposure. However, without dose estimates and a dose-response model, and without information on cancer pre-disposing conditions, it is likely that that excess cancer risks following CT scans have been inflated by bias from both reverse causation and confounding by indication. We look forward to future results from this study, with longer follow-up times.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1The number of CT exposed individuals (and cancer cases) were presented for a two-year lag. There were 1,444 cancer cases in 1,179,021 CT exposed patients.
2The main analysis used a one-year lag period. Here, there were 3,150 cases among 680,211 CT exposed patients.
3The UK study observed 74 leukaemia cases across 1,720,984 person-years and 135 brain cancer cases across 1,188,207 person-years (Table 2 in Pearce et al.).
4Cancer rates in the South Korean study were estimated using only children exposed low-dose diagnostic radiation to mimic the UK study, which did not have an unexposed population. The number of cancer cases and person-years were extracted from Tables 3 and 5 (respectively) in Hong et al. There were 332 leukaemia and myelodysplasia cases and 183 brain cancer cases across 4,499,100 person-years. The UK study used a five-year lag for brain cancer; however, brain cancer cases were only presented for a two-year lag in the South Korean study.
References
- Hong JY, Han K, Jung JH, Kim JS. Association of Exposure to Diagnostic Low-Dose Ionizing Radiation With Risk of Cancer Among Youths in South Korea. JAMA Netw Open 2019;2:e1910584. [Crossref] [PubMed]
- Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Craft AW, Parker L, Berrington de Gonzalez A. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380:499-505. [Crossref] [PubMed]
- Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013.346. [PubMed]
- Huang WY, Muo CH, Lin CY, Jen YM, Yang MH, Lin JC, Sung FC, Kao CH. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer 2014;110:2354-60. [Crossref] [PubMed]
- Krille L, Dreger S, Schindel R, Albrecht T, Asmussen M, Barkhausen J, Berthold JD, Chavan A, Claussen C, Forsting M, Gianicolo EA, Jablonka K, Jahnen A, Langer M, Laniado M, Lotz J, Mentzel HJ, Queisser-Wahrendorf A, Rompel O, Schlick I, Schneider K, Schumacher M, Seidenbusch M, Spix C, Spors B, Staatz G, Vogl T, Wagner J, Weisser G, Zeeb H, Blettner M. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys 2015;54:1-12. [Crossref] [PubMed]
- Journy N, Rehel JL, Ducou Le Pointe H, Lee C, Brisse H, Chateil JF, Caer-Lorho S, Laurier D, Bernier MO. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer 2015;112:185-93. [Crossref] [PubMed]
- Meulepas JM, Ronckers CM, Smets AMJB, Nievelstein RAJ, Gradowska P, Lee C, Jahnen A, van Straten M, de Wit MY, Zonnenberg B, Klein WM, Merks JH, Visser O, van Leeuwen FE, Hauptmann M. Radiation Exposure From Pediatric CT Scans and Subsequent Cancer Risk in the Netherlands. J Natl Cancer Inst 2019;111:256-63. [Crossref] [PubMed]
- Berrington de Gonzalez A, Salotti JA, McHugh K, Little MP, Harbron RW, Lee C, Ntowe E, Braganza MZ, Parker L, Rajaraman P, Stiller C, Stewart DR, Craft AW, Pearce MS. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer 2016;114:388-94. [Crossref] [PubMed]
- Journy N, Roué T, Cardis E, Le Pointe HD, Brisse H, Chateil JF, Laurier D, Bernier MO. Childhood CT scans and cancer risk: impact of predisposing factors for cancer on the risk estimates. J Radiol Prot 2016;36:N1-7.
- Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295-300. [Crossref] [PubMed]
- Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed. Blackwell Science; 2003.
- Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 2016;31:337-50. [Crossref] [PubMed]
- Greenland S, Michels KB, Robins JM, Poole C, Willett WC. Presenting Statistical Uncertainty in Trends and Dose-Response Relations. Am J Epidemiol 1999;149:1077-86. [Crossref] [PubMed]
- Arbogast PG. Performance of floating absolute risks. Am J Epidemiol 2005;162:487-90. [Crossref] [PubMed]