
Nephrogenic systemic fibrosis: the end of the story?
Gadolinium-based contrast agents (GBCA) have been standardly intravenously applied to improve visibility of MRI examinations since 1988, when gadopentetate dimeglumine (Magnevist®) was approved for clinical practice. Gadolinium itself is a heavy metal, highly toxic in biological systems. Therefore, gadolinium must be bound to ligands for the purpose of contrast agents. GBCA consist of gadolinium ion and a chelating molecule, which could be cyclic or linear. The macrocyclic molecule pre-organizes the rigid ring to cage the gadolinium ion, which offers better protection and binding of gadolinium ion. Linear chelates with a flexible open chain are less stable and provide weaker link of the gadolinium ion. The rate of gadolinium dissociation from the macrocyclic ligands is remarkably slower than their dissociation from the linear chelates (1).
Nephrogenic systemic fibrosis (NSF) is a disease associated with GBCA applications and connected to impaired kidney function. NSF was firstly described by Cowper et al. in 2000 (2). It is necessary to mention that Cowper et al. did not link that disease to gadolinium applications (2). This was done several years later (in 2006) by a group of nephrologists led by Grobner and Marckmann (3,4). NSF represents a systemic multiorgan disorder characterized by fibrosis. It is a serious and possibly life-threatening disease, affecting not only skin, but all fibrous tissues in the body including those in internal organs such as heart, liver, lungs and muscles. The failure of these organs may be responsible for patients’ death. However, NSF is a highly clinically variable disease, some patients may suffer only from mild cosmetics skin affection, others may be limited in the movement and even in daily self-care and in rare cases the disease is lethal. Absolute majority of NSF cases have been associated with linear GBCA (5).
Grobner’s and Marckmann’s research (3,4) broke down the common unfortunate illusion about the general safeness of GBCA. The reaction of authorities and radiology societies was prompt. Food and Drug Administration, European Medicines Agency, American College of Radiology, European Society of Urogenital Radiology and other societies released warnings, guidelines and recommendations how to correctly use GBCA in daily medical practice and how to manage patients with renal insufficiency (6-9). According to them, using high risk linear GBCA is not recommended in patients with the estimated glomerular filtration rate (eGFR) under 30 mL/min/1.73 m2 including hemodialysis patients nor in patients suffering from acute kidney insufficiency. In the Tables 1 and 2 you can find more details about characterization of GBCA including their NSF risk and recommended management. In 2011 Zou et al. reviewed 370 biopsy-proven NSF cases (5). According to their findings no NSF case had been reported 3 years before their paper was published, thus since 2008 (5). In the last decade we have been thinking that the number of NSF cases has been limited and we have been living in the world where NSF has been eradicated.
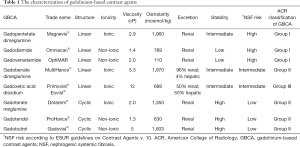
Full table

Full table
Recently, Attari et al. published the remarkable paper “A systematic Review of 639 Patients with Biopsy-confirmed Nephrogenic Systemic Fibrosis” in Radiology [2019] (10). They searched PubMed from January 2000 to February 2019 according to the key words “Nephrogenic systemic fibrosis”, “NSF” and “Nephrogenic fibrosing dermopathy”. Furthermore, they extracted the risk factors, clinical data, outcomes and possible treatment in patients with biopsy-verified NSF. They included 639 affected patients from 173 articles. The number of affected subjects was nearly double in comparison to the last above-mentioned review by Zou et al. (5). Affected subjects were almost equally males and females, aged 48±16 years. NSF was manifested only by dermatologic changes in 29% of them and motion limitation was reported in 71% of cases. Involvement of internal organs was found in 56% of patients and in 47.5% of cases some autoimmune disease was also reported. About 97% of included subjects had a history of GBCA exposure. The majority of cases were associated with the application of Gadodiamide (approximately 76%), the second most common cases were associated with Gadopentetate dimeglumine (12%). The majority of subjects (86%) were on dialysis around the time of GBCA applications; 4/5 of reported patients suffered from chronic kidney failure, 1/5 of cases from acute kidney failure. Unfortunately, clinical outcomes or follow-ups were reported only in 341 patients, 110 of them died; however, only in 4 of them their death was attributed directly to NSF. Partial resolution of NSF symptoms was found in 84 patients and even cure of NSF was reported in 12 patients; 40% of them all had acute kidney failure. Improvement of the disease was also reported in females during pregnancy (in 22 subjects) and in some patients after the cessation of ß-blockers (in 5 subjects). Approximately 18% of cases were reported as a stable disease.
Finally, Attari et al. (10) estimated the rate of NSF cases per million exposures. Firstly, they assumed equal market share for high risk (Group I) and low risk (Group II) GBCA (see also Tables 1 and 2) and came to the rate of 1.52 per million exposures for high risk versus 0.008 per million exposures for low risk of GBCA. Secondly, they counted the rate of NSF cases per million exposures according to market share sensitivity analysis (90% of market share for high risk GBCA) and came to the rate of 0.84 to 0.04 cases per million exposures (thus, 20-fold higher rate of NSF for high risk GBCA). By assuming equal use of GBCA before versus after the implementation of warnings, regulatory restrictions and recommendations, the rate of NSF per million exposures decreased from 2.07 before 2008 to 0.028 after 2008. It is necessary to mention the fact, that Attari et al. could not determine or estimate the number of GBCA exposures in high-risk subjects (10).
I would like to highlight some findings reported by Attari et al. (10). First, they found 14 cases of NSF without any previous intravenous applications of GBCA. Zou et al. in the previous review [2011] also reported even 8% of cases without clear previous GBCA exposures (5). Both papers support the hypothesis that GBCA can trigger NSF; however, there are probably also other triggers, which are not completely known. The authors bring possibilities of the influence of ß-blockers, hyperphosphatemia, acidosis, epoetin or proinflammatory events. The second interesting finding is that NSF looks like an age dependent disease, because it was not reported in newborns and toddlers; the youngest reported child was 6 years old. This fact is remarkable, especially when newborns with immature kidneys were often examined with high doses of GBCA. Also, affection of the patients above 80 years were exceptional, only 7 cases were reported by Attari et al. The similar findings were also published by Zou et al. in 2011 (5). We can only hypothesize the reasons of these findings; maybe the less active immune system may be responsible for the protection of young children against NSF. Finally, and the most important, only seven NSF cases after GBCA exposures were reported since 2008. This result confirms the huge success of regulations, warnings and recommendations of authorities and radiology societies. It shows the power of recent modern medicine, its flexibility and abilities to fight with emerged risks and threats and effectively prevent new cases. However, I am in temptation to raise the “devil question”: “Do we not deny access to the contrast enhanced MRI to patients with kidney disease, who need it?” From my medical practice I have repeatedly witnessed situations when contrast enhanced MRI was cancelled in patients with chronic kidney disease (although eGFR did not reach the cut-off limit) due to the fear of NSF. I must also mention that in European Union only low risk GBCA are available for medical practice in these days, because European Medical Agency has suspended intravenous use of all high risk GBCA from the European market (9).
It is understandable, that the recent work by Attari et al. (10) included severe confounding bias in all articles and substantial number with selection bias, missing data bias, limited outcomes and conflicts of interest. Authors honestly stated the limitations of their paper and discussed them correctly. On the other hand, they clearly showed that the number of NSF cases has been much higher that we had supposed. I think, today it is without doubts that their estimated rate of NSF cases per million exposures is probably underestimated. They reviewed only biopsy-proven NSF cases that were published in the journals indexed on PubMed. I am convinced that there must be cases which were suspected and were not sampled for different reasons, or were sampled and proven and published in some regional non-indexed journals or were not published due to stigma of gadolinium toxicities. Moreover, some cases may be/must be underdiagnosed and unrecognized. Next questions, which are not definitely solved, are: “Do low-risk GBCA really cause NSF? Is the risk of their application higher than the risk of avoiding them from diagnostic process?” Honestly, in these days, I do not see the way for answering these questions.
The other important message of this paper is some kind of general warning. However, this message has also two different faces. On one side, this article reminds us that no drug in human modern medicine is without side effects. Since 1988 to 2006 (18 years!), GBCA were considered generally safe and were used liberally in patients with renal functions impairment, hemodialysis patients included. Very often subjects suffering from renal impairment were preferentially sent for enhanced MRI examinations to avoid CT with iodine contrast administration. That approach was later shown as very unfortunate. However, it seems that gadolinium story has not been finished yet. In 2014, Kanda et al. published a study, which suggested gadolinium deposition in the brain structures after repeated GBCA applications in subjects with healthy kidneys (11) and their suspicion was immediately confirmed histologically (12). Since that time, a lot of scientific papers have been dealt with gadolinium depositions elsewhere in the human or animal bodies (13). However, I must stress the fact, that substantial part of those papers bring a huge amount of bias and conflicts of interest. Semelka et al. even proposed the new “gadolinium deposition disease” and their results were based on questioning the anonymous participates from “MRI-Gadolinium-Toxicity Support Group”, which is a group of patients, their friends and families active on social networks (14). It is necessary to clearly state that the clinical effect of those gadolinium depositions is still unclear and no direct side effects have been proven. I do not say that those gadolinium depositions in the brain and in other organs must be without side effects, but the mess and sometimes panic around the gadolinium does not serve to anybody and may cause the “collateral” medical damages for our patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Rofsky NM, Sherry AD, Lenkinski RE. Nephrogenic systemic fibrosis: A chemical perspective. Radiology 2008;247:608-12. [Crossref] [PubMed]
- Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000;356:1000-1. [Crossref] [PubMed]
- Grobner T. Gadolinium--a specific trigger for development of nephrogenic fibrosis dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104-8. [Crossref] [PubMed]
- Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17:2359-62. [Crossref] [PubMed]
- Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR. Nephrogenic systemic fibrosis: review of 370 biopsy-confirmed cases. JACC Cardiovasc Imaging 2011;4:1206-16. [Crossref] [PubMed]
- U.S. Food and Drug Administration. FDA requests boxed warning for contrast agents used to improve MRI images. Available online: http://wayback.archiveit.org/7993/20170112033008/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108919.htm, accessed July 25, 2019.
- U.S. Food and Drug Administration. FDA Safety Communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm223966.htm, accessed July 25, 2019.
- ACR Manual on Contrast Media Version 10.3. ACR Committee on Drugs and Contrast Media. Available online: https://www.acr.org/Clinical-Resources/Contrast-Manual, accessed July 25, 2019.
- ESUR guidelines on contrast agents v.10. European Society of Urogenital Radiology. Available online: http://www.esur-cm.org, accessed July 25, 2019.
- Attari H, Cao Y, Elmholdt TR, Zhao Y, Prince MR. A Systematic Review of 639 Patients with Biopsy-confirmed Nephrogenic Systemic Fibrosis. Radiology 2019;292:376-86. [Crossref] [PubMed]
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834-41. [Crossref] [PubMed]
- Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015;276:228-32. [Crossref] [PubMed]
- Holesta M, Weichet J, Grilli Wagnerova M, Lukavsky J, Malikova H. Gadoxetate disodium, a modern hepatospecific MRI contrast agent: Indirect signs for gadolinium deposition in the brain structures with signal intensities increase after intravenous applications. Neurol India 2018;66:1771-5. [Crossref] [PubMed]
- Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magnetic Resonance Imaging 2016;34:1383-90. [Crossref] [PubMed]


