
Whole-tumor perfusion CT using texture analysis in unresectable stage IIIA/B non-small cell lung cancer treated with recombinant human endostatin
Introduction
Lung cancer is the most common type of cancer in China in terms of incidence and mortality (1). Currently, concurrent chemoradiotherapy (CCRT) is considered as the standard treatment for patients with locally advanced non-resectable non-small cell lung cancer (NSCLC) (2,3) However, the clinical outcomes of these patients still remain poor, with a 5-year survival rate of approximately 20% (4). At present, several studies have indicated that anti-angiogenic therapies can improve the effect of chemoradiotherapy, while the potential underlying mechanism of this effect could be related to the normalization of tumor vasculature, alleviating tumor hypoxia (5). Therefore, anti-angiogenic therapy combined with chemoradiotherapy has been considered as a new generation treatment strategy for locally advanced NSCLC patients. Recombinant human endostatin (rhES) (Endostar), an endogenous inhibitor of angiogenesis, has been proven to normalize the tumor vasculature, improve the blood supply, and increase the sensitivity of radiation, thus improving the response rate and survival in advanced NSCLC patients (6,7).
Over the last decade, several studies have demonstrated that lung tumor vascularity can be evaluated using perfusion computed tomography (CT). According to Tacelli et al. (8), the early changes in lung cancer vascularity under anti-angiogenic chemotherapy may help predict therapeutic response. Conventionally, perfusion CT has been performed as an axial technique at a single anatomical level; however, the tumor vasculature is heterogeneous both structurally and functionally, and currently whole-tumor analysis is available by sufficiently wide detectors. Ng et al. (9) reported that the whole tumor perfusion CT measurement was more repeatable, compared to conventional single tumor level evaluations. CT texture analysis (CTTA) is an image processing technique that can be applied to routinely acquire images to provide quantitative information about tumor heterogeneity that reflect the characteristics, variation, and distribution within the tumor (10). This allows for a more detailed and quantitative assessment of the tumor features than visual analysis. A growing amount of evidence has demonstrated the ability of texture analysis to predict treatment response and survival as it can quantitatively reflect tumor metabolism, angiogenesis, or hypoxia in patients with NSCLC (11-14).
Hence, this study aimed to observe the dynamic changes of blood perfusion with whole-tumor CT perfusion imaging using texture analysis in patients with unresectable stage IIIA/B NSCLC treated with Endostar. Our hypothesis was that whole-tumor CT perfusion imaging using texture analysis can demonstrate the increasing tumor perfusion in patients who undergo treatment with Endostar. To our knowledge, this is the first study to explore the use of texture analysis using whole-tumor perfusion CT to predict the response of locally advanced unresectable NSCLC treated with antiangiogenic therapy.
Methods
This study was approved by the ethics committee of Cancer Hospital of Zhejiang Province and written informed consent was obtained from all patients. This is a multi-center phase II single-arm clinical trial, which was registered with ClinicalTrials.gov (number NCT01733589).
Patient population
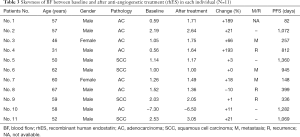
From May 2014 to May 2015, 11 patients diagnosed with locally advanced unresectable NSCLC were recruited. All patients underwent contrast-enhanced perfusion CT at baseline and a second CT scan 1 week after anti-angiogenic therapy (Endostar). Of these 11 cases, 9 were men and 2 were women, 4 had squamous cell carcinoma and 7 had adenocarcinoma, and the median age was 57 years (ranging from 31 to 67 years). According to the AJCC TNM classification for NSCLC, 7 cases were stage IIIA and 4 cases were stage IIIB.
Treatment procedure
All patients received Endostar (7.5 mg/m2/24 h) by continuous pumping into the vein for 5 days at weeks 1, 3, 5 and 7. Patients received thoracic 3D conformal radiation at 60–66 Gy in 30–33 fractions for 6 to 7 weeks, concurrent with 2 cycles of etoposide 50 mg/m2 on days 1–5 and 29–33 and cisplatin 50 mg/m2 on days 1, 8, 29 and 36.
Data acquisition
CT examinations were performed using a 64-slice CT scanner (Somatom Definition Flash). Initially, a non-contrast chest scan was performed to localize the tumor. On the basis of this scan, a fixed scan range of 100 mm in the direction of z-axis was determined for perfusion CT, covering the whole tumor. The following perfusion parameters were applied: 80 kV, 60 mAs, with a slice thickness of 3 mm. Forty-five mL of iopromide (370 mg/mL iodine) was injected with a flow rate of 5 mL/s, followed by a flush of 45 mL NaCl at 5 mL/s. Whole-tumor perfusion CT acquisition started with a 2 s delay after the injection, and a total of 30 scans with 1.5 s temporal sampling was done. Images were reconstructed at a slice thickness of 3 mm with a 2 mm increment. Re-examination of perfusion CT was repeated and the data were compared 1 week after treatment initiation with Endostar.
Data analysis
All CT images were anonymized and then transferred to a post-processing workstation equipped with Volume Perfusion CT Body software (VPCT) (Siemens Healthcare, Germany). For the arterial input function (AIF), a region of interest (ROI) was drawn inside the aorta. Each slice along the z-axis of the entitled tumor was evaluated by freehand outlining an ROI within the border of the tumor, thus obtaining the entire tumor volume. This procedure was conducted by two experienced radiologists (L Shi and XL Zhou), similarly to what was done previously (15). The software generated three-dimensional color-coded maps according to the following perfusion parameters: blood flow (BF, mL/100 mL/min), blood volume (BV, mL/100 mL), and permeability (PMB, mL/100 mL/min). The above parameters were imported into the OmniKinetics software (GE Healthcare, USA). The texture parameters of BF, BV, and PMB within the tumor ROI were quantitatively assessed, including for values of skewness, kurtosis, and entropy (a measure of irregularity). Skewness represents the degree of asymmetric distribution in the image histogram. If it is positive, the distribution is positively skewed (more values are lower values), and if it is negative, the distribution is negatively skewed (more values are higher values). If it is 0, then the distribution is symmetrical. Kurtosis is a measure of whether the data are heavy-tailed or light-tailed relative to the normal distribution, and the high values of kurtosis tend to have heavy tails, or outliers. The entropy measures the randomness of the distribution of the coefficient values over the intensity levels, and a high value of entropy means that the distribution is among the more intense levels in the image. The intraclass correlation (ICC) coefficients for each parameter were >0.85 between L Shi and XL Zhou.
Statistical analysis
Statistical analyses were performed using statistical software (SPSS version 19.0, Chicago, USA), and for all other comparisons. P values of less than 0.05 were considered to be statistically significant. Continuous variables are presented as mean ± standard deviation (SD). Comparisons of perfusion and textural parameters between baseline and after anti-angiogenic treatment were performed using Wilcoxon signed-rank test. Progression-free survival (PFS) was calculated from the date of initiation of Endostar treatment to the date of objective tumor progression, or until the date of death due to any cause, or last follow-up time, whichever occurred first. The associations of continuous variables with PFS were evaluated using the Cox proportional hazards regression model.
Results
Comparison between baseline and after anti-angiogenic treatment rhES regarding perfusion CT imaging
Table 1 comprises the mean values of BF, BV, and PMB at baseline and after anti-angiogenic treatment. Blood perfusion change was observed but there were no statistical differences in regards to the mean BF, BV, and PMB values (P=0.594, 0.477 and 0.328, respectively).

 ± s) (N=11)
± s) (N=11) Full table
Comparison between baseline and after anti-angiogenic treatment (rhES) regarding textural analysis features
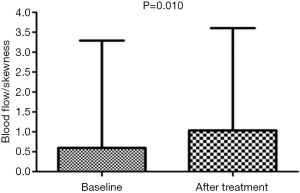
The skewness of BF showed significant differences between baseline and after treatment values (0.6±2.7 vs. 1.0±2.6, respectively, P=0.010) (Figure 1, Tables 2,3), while skewness of BV and PMB showed no significant variation (P=0.477 and 0.213, respectively). The kurtosis and entropy for BF, BV, and PMB showed no significant differences (all P>0.05). In adenocarcinoma, the mean values of BF showed no significant differences at baseline and after treatment (76.5±25.7 vs. 101.2±46.4, respectively, P=0.398), while the values of skewness for BF were significantly higher after treatment than at baseline (−0.19±3.3 vs. 0.59±3.2, respectively, P=0.028). Furthermore, the pixel values of BF in one adenocarcinoma patient were significantly higher after treatment than at baseline (61.1±29.1 vs. 72.3±41.6, respectively, P<0.001) (Figure 2). The increased BF value was demonstrated in the BF map (Figure 2). In squamous carcinoma, both the mean values of BF (96.8±42.6 vs. 93.5±43.9, P=0.068) and the skewness for BF (1.7±0.73 vs. 1.8±0.94, P=0.068) showed no significant differences at baseline than after treatment, respectively.

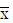 ± s) (N=11)
± s) (N=11) Full table
Full table
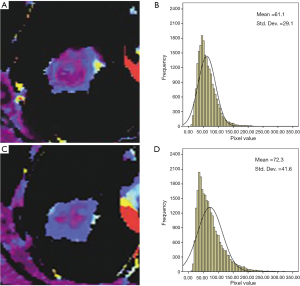
Association between perfusion CT imaging parameters and PFS
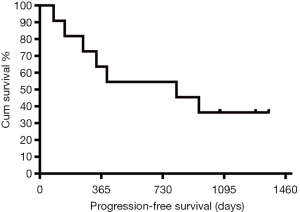
Of the 11 patients, 6 patients showed disease progression, including primary tumor sites on the spleen, intra-lung area, and pericardium. One patient died during the follow-up period. The median PFS was 812 days (Figure 3). In univariate Cox regression, the correlation of the mean values, skewness, kurtosis, and entropy of BF, BV, and PMB at baseline and after anti-angiogenetic treatment with PFS was not significant (all P>0.05). Meanwhile, the changes in these parameters also showed no significant association with PFS (all P>0.05).
Discussion
Endostatin, a carboxy-terminus fragment of collagen XVIII, was first identified as an antiangiogenic molecule in 1997 by O’Reilly et al., and was understood to specifically inhibit endothelial proliferation and potently inhibit angiogenesis and tumor growth (16). Endostar purified in Escherichia coli was approved by the State Food and Drug Administration (SFDA) of China for the treatment of NSCLC in 2005 (17). Ling et al. (18) demonstrated that Endostar exerted antiangiogenic effects via blocking the vascular endothelial growth factor (VEGF)-induced tyrosine phosphorylation of vascular endothelial growth factor receptor (VEGFR-2) of endothelial cells as well as overall VEGFR-2 expression. Moreover, Jiang et al. (6) confirmed that Endostar has a “time window” of vascular normalization in human NSCLC; i.e., within about 1 week after administration, and when combined with concurrent radiotherapy, the “time window” may have synergistic therapeutic effects. During the last decade, several studies have shown that the combination of Endostar with radiotherapy or chemotherapy successfully improves treatment response and prolongs survival in advanced NSCLC (6,7,19,20).
Perfusion CT imaging is a useful tool in the field of cancer imaging to evaluate disease response or progression after treatment (8,9,21,22). Few studies have investigated the role of CTTA in lung cancer imaging. In our study, there were no statistical differences with regard to the mean values of BF, BV, and PMB. However, improved blood perfusion could be found when referring to the absolute values of BF and BV (mean ± SD, BF: baseline 83.9±32.3 mL/100 mL/min, after treatment: 98.4±43.4 mL/100 mL/min; BV: baseline 93.6±37.2 mL/100 mL, after treatment 103.7±26.2 mL/100 mL). Using texture analysis, we found that the skewness for BF showed significant differences at baseline and after treatment, while the skewness of BV and PMB showed no significant variation. As is known, the skewness reflects the degree of asymmetry in the histogram distribution, and if the treatment had been effective, the absolute values of the skewness would have been higher. Additionally, the mean values of BF represented the overall level of tumor BF; however, the skewness was a more comprehensive indicator for BF, which considers the influence of tumor heterogeneity among NSCLC. This might be the reason as to why the mean values of BF were not significantly different before and after treatment with Endostar, while the skewness was significant. The values of kurtosis as well as entropy for BF, BV, and PMB showed no significant differences. These results suggest that the texture analysis based on perfusion CT imaging was superior to the routine perfusion CT measurements in locally advanced unresectable NSCLC treated with Endostar.
Approximately 85% of newly diagnosed lung cancers are NSCLC, while approximately 30% are squamous carcinomas with a worse survival rate than adenocarcinoma (23). Previous published studies demonstrated that Endostar could improve the survival of patients in both squamous carcinoma and adenocarcinoma. In our study, significant BF improvement was found only in adenocarcinoma patients, and not in squamous carcinoma patients. This indicates that adenocarcinoma was more sensitive than squamous in NSCLC patients treated with Endostar, which is consistent with a previous study (24).
All perfusion CT imaging parameters showed no significant association with PFS, and the size of the series might be the most important reason. However, several cases should be taken into consideration. All four patients without progression were male, and their increasing skewness of BF showed varying degrees. The PFS of female patients in this study was significantly shorter than male patients. We hope that larger sample studies can verify if male patients with NSCLC whose skewness of BF shows increasing values may benefit from the treatment of continuous infusion of Endostar combined with concurrent etoposide plus cisplatin and radiotherapy.
Notably, the current study had several limitations. First, the size of the patients was small, precluding the detailed analysis of treatment response in NSCLC patients treated with Endostar. Second, all cases were recruited into a multi-center phase II single-arm clinical trial, and so no comparison between Endostar plus CCRT and CCRT alone was established in this study. Also, the dynamic changes of blood perfusion could not evaluate the duration of therapy by CCRT. A large sample size is warranted to verify our preliminary results. Moreover, the biological markers that refer to the tumor vasculature and hypoxia status such as microvessel density, vascular endothelial growth factor, and hypoxia-inducible factor within the NSCLC tumors treated with Endostar, should also be included in future study to explore the underlying mechanism.
Conclusions
In summary, an improvement in blood perfusion could be observed by whole-tumor perfusion CT using texture analysis in patients with stage IIIA/B NSCLC treated by Endostar, especially in adenocarcinoma patients. Texture analysis based on whole-tumor perfusion CT imaging can assist in evaluating the therapeutic response that seems to be superior to routine perfusion CT measurements. The textural analysis based on perfusion CT images could represent a very useful non-invasive tool for clinicians to manage anti-angiogenic therapies more efficiently if confirmed by larger studies.
Acknowledgments
Funding: This study was supported by a grant from the Zhejiang Medical and Technology Foundation (NO. 201480784), and the Zhejiang Provincial 151 Talent Project (L Shi).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Cancer Hospital of Zhejiang Province and written informed consent was obtained from all patients.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR. American Society of Clinical Oncology. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330-53. [Crossref] [PubMed]
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P, Pecen L. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 2004;46:87-98. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Bao Y, Peng F, Zhou QC, Yu ZH, Li JC, Cheng ZB, Chen L, Hu X, Chen YY, Wang J, Wang Y, Ma HL, Xu ZM, Lu RB, Deng XW, Chen M. Phase II trial of recombinant human endostatin in combination with concurrent chemoradiotherapy in patients with stage III non-small-cell lung cancer. Radiother Oncol 2015;114:161-6. [Crossref] [PubMed]
- Jiang XD, Dai P, Qiao Y, Wu J, Song DA, Li SQ. Clinical study on the recombinant human endostatin regarding improving the blood perfusion and hypoxia of non-small-cell lung cancer. Clin Transl Oncol 2012;14:437-43. [Crossref] [PubMed]
- Han BH, Xiu QY, Wang HM, Shen J, Gu AQ, Luo Y, Bai CX, Guo SL, Liu WC, Zhuang ZX, Zhang Y, Zhao YZ, Jiang LY, Shi CL, Jin B, Zhou JY, Jin XQ. A multicenter, randomized, double-blind, placebo-controlled safety study to evaluate the clinical effects and quality of life of paclitaxel-carboplatin (PC) alone or combined with endostar for advanced non-small cell lung cancer (NSCLC). Zhonghua Zhong Liu Za Zhi 2011;33:854-9. [PubMed]
- Tacelli N, Santangelo T, Scherpereel A, Duhamel A, Deken V, Klotz E, Cortot A, Lafitte JJ, Wallyn F, Remy J, Remy-Jardin M. Perfusion CT allows prediction of therapy response in non-small cell lung cancer treated with conventional and anti-angiogenic chemotherapy. Eur Radiol 2013;23:2127-36. [Crossref] [PubMed]
- Ng QS, Goh V, Milner J, Sundin J, Wellsted D, Saunders MI, Hoskin PJ. Quantitative helical dynamic contrast enhanced computed tomography assessment of the spatial variation in whole tumour blood volume with radiotherapy in lung cancer. Lung Cancer 2010;69:71-6. [Crossref] [PubMed]
- Ahn SJ, Kim JH, Park SJ, Han JK. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis. Eur J Radiol 2016;85:1867-74. [Crossref] [PubMed]
- Miles KA. How to use CT texture analysis for prognostication of non-small cell lung cancer. Cancer Imaging 2016;16:10. [Crossref] [PubMed]
- Hayano K, Kulkarni NM, Duda DG, Heist RS, Sahani DV. Exploration of Imaging Biomarkers for Predicting Survival of Patients With Advanced Non-Small Cell Lung Cancer Treated With Antiangiogenic Chemotherapy. AJR Am J Roentgenol 2016;206:987-93. [Crossref] [PubMed]
- Lovinfosse P, Janvary ZL, Coucke P, Jodogne S, Bernard C, Hatt M, Visvikis D, Jansen N, Duysinx B, Hustinx R. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 2016;43:1453-60. [Crossref] [PubMed]
- Ohri N, Duan F, Snyder BS, Wei B, Machtay M, Alavi A, Siegel BA, Johnson DW, Bradley JD, DeNittis A, Werner-Wasik M, El Naqa I. Pretreatment 18F-FDG PET Textural Features in Locally Advanced Non-Small Cell Lung Cancer: Secondary Analysis of ACRIN 6668/RTOG 0235. J Nucl Med 2016;57:842-8. [Crossref] [PubMed]
- Ng CS, Wei W, Ghosh P, Anderson E, Herron DH, Chandler AG. Observer Variability in CT Perfusion Parameters in Primary and Metastatic Tumors in the Lung. Technol Cancer Res Treat 2018;17:1533034618769767. [Crossref] [PubMed]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277-85. [Crossref] [PubMed]
- Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res 2006;312:594-607. [Crossref] [PubMed]
- Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, Chen Y, Guo QL. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun 2007;361:79-84. [Crossref] [PubMed]
- Jiang XD, Dai P, Wu J, Song DA, Yu JM. Effect of recombinant human endostatin on radiosensitivity in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:1272-7. [Crossref] [PubMed]
- Wang J, Sun Y, Liu Y, Yu Q, Zhang Y, Li K, Zhu Y, Zhou Q, Hou M, Guan Z, Li W, Zhuang W, Wang D, Liang H, Qin F, Lu H, Liu X, Sun H, Zhang Y, Wang J, Luo S, Yang R, Tu Y, Wang X, Song S, Zhou J, You L, Wang J, Yao C. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi 2005;8:283-90. [PubMed]
- Kiessling F, Boese J, Corvinus C, Ederle JR, Zuna I, Schoenberg SO, Brix G, Schmähl A, Tuengerthal S, Herth F, Kauczor HU, Essig M. Perfusion CT in patients with advanced bronchial carcinomas: a novel chance for characterization and treatment monitoring? Eur Radiol 2004;14:1226-33. [Crossref] [PubMed]
- Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, Mueller PR, Lee TY. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 2005;234:785-92. [Crossref] [PubMed]
- Heist RS, Mino-Kenudson M, Sequist LV, Tammireddy S, Morrissey L, Christiani DC, Engelman JA, Iafrate AJ. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol 2012;7:1775-80. [Crossref] [PubMed]
- Bevilacqua A, Gavelli G, Baiocco S, Barone D. CT Perfusion in Patients with Lung Cancer: Squamous Cell Carcinoma and Adenocarcinoma Show a Different Blood Flow. Biomed Res Int 2018;2018:6942131. [Crossref] [PubMed]


