Utility of ADC measurement on diffusion-weighted MRI in differentiation of prostate cancer, normal prostate and prostatitis
Introduction
Prostate cancer is most frequently encountered tumor of men (1). The prognosis of prostate cancer mostly depends on early diagnosis. Rectal examination, measurement of prostate specific antigen (PSA) and transrectal ultrasound (TRUS) have been used to detect prostate cancer at early phase.
Diffusion weighted magnetic resonance imaging (DWMRI) is based on the molecular diffusion of water molecules in biological tissues (2). Apparent diffusion coefficient (ADC) value is a quantitative parameter of DWMRI representing water diffusion in extracellular and extravascular space and capillary perfusion (3). ADC values have been shown to be decreased in various malignancies of different organs due to hypercellularity (4-7). Recent studies concluded that ADC measurement on DWMRI can differentiate malignant prostate lesions from benign prostatic tissue (8-11).
In this study, our aim was to investigate the role of ADC measurement in differentiation between prostate cancer, normal prostate parenchyma and prostatitis at low (b 100 s/mm2), intermediate (b 600 s/mm2) and high (b 1,000 s/mm2) diffusion gradients.
Materials and methods
Patient group
Fifty patients (age range, 50-85 years old; mean age, 67 years old) presented with the suspicion of prostate cancer according to abnormal digital rectal examination and increased PSA levels were included in this study. Dynamic contrast enhanced MRI and DWMRI followed by TRUS guided biopsy were performed in all patients.
MRI protocol
Dynamic contrast enhanced MRI and DWMRI of patients were performed with 1.5 T GE Signa Hispeed Excite MR System (General Electric, Milwaukee, WI). MRI examinations were obtained with body coil in the supine position. In all MRI examinations prostate was localized in the center of the 4-channel TORSO or spine coil. The MRI protocol included axial and coronal T1- and T2-weighted images, axial and coronal T1-weighted images after intravenous contrast agent administration and DW images obtained at b 100, 600 and 1,000 s/mm2 gradients. The parameters of DWMRI examinations were as follows: matrix, 128×128; NEX, 1.0; FOV, 20; slice thickness, 5 mm; slice gap, 0; diffusion direction, all directions; TR, 8,000 ms; TE, 80 ms; mean region of interests (ROI), 45 mm2. ADC maps obtained from DW images at b 100, b 600 and b 1,000 s/mm2 gradients.
Analysis of the MR images and ADC measurement
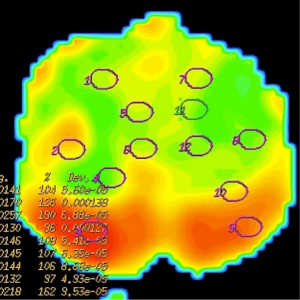
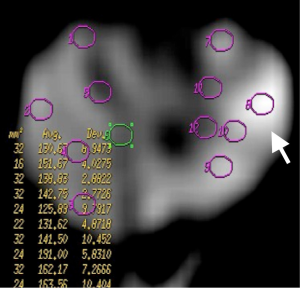
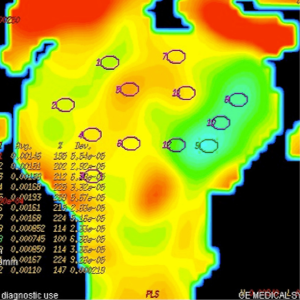
ADC measurements of prostate were done on 3 separate levels (apical gland, midgland and basal gland) in the prostate of the patients. Twelve quadrants visualized as suspicious on T2-weighted images on each three gland level was used to measure ADC value constituting a total of 36 ADC value measurement at all three diffusion gradients in each patient. Twelve quadrant measurements were intended to be obtained from the localizations that biopsy specimens were obtained. The ADC values were measured by insertion of ROI which have the mean area of 45 mm2. ADC measurements were performed on color-coded ADC maps automatically after calculating the diffusion difference between each gradient (b 100, 600 and 1,000 s/mm2) and the b 0 gradient on a workstation (Advantage Windows, software version 2.0, General Electric Medical Systems). Monoexponential method was used in ADC measurements. A minimum mean square error estimator was used in monoexponential method to minimize the mean square error of the fitted ADC values.
Pathologic findings
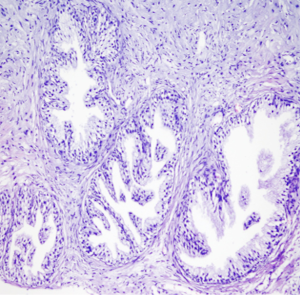
Prostatectomy specimens were sliced between the surgical boundaries of proximal and distal urethra. Prostatectomy specimens were examined with 5 mm thick slices in accordance with 5 mm thick DW images. By this method, we aimed to make ADC measurement from the points as possible same with the pathological examination localizations.
The ADC values of benign and malignant prostate lesions were compared with the histopathologic results of prostatectomy and biopsy specimens. Patients with benign prostate lesions, prostatitis and prostate cancer were classified as group I, II and III, respectively.
Statistical analysis
Statistical analysis was performed with SPSS (Statistical Package for the Social Sciences) 10.0 for Windows programme. ADC values were defined as mean ± standard deviation. Student t test, and ROC analysis tests were used to compare ADC values of group I, II and III patients at b 100, 600 and 1,000 s/mm2 gradients. The differences in the ADC values were considered to be statistically significant when the P value was <0.05.
Results
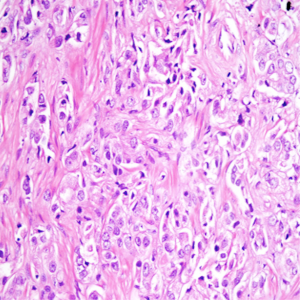
Histopathologic examination of TRUS guided biopsy results of 50 patients revealed 30 adenocarcinoma, 11 normal prostate parenchyma and 9 prostatitis. Radical prostatectomy was performed in 10 patients who were diagnosed as adenocarcinoma while 20 patients with prostate carcinoma were treated with medical treatment and transurethral resection (TUR) of prostate. Patients with prostatitis were treated with drug therapy.
Prostate cancers manifested with intense enhancement at arterial phase and exhibited wash-out at late phase on dynamic contrast enhanced MR images. Early enhancement with heterogeneous appearance and patchy pattern was observed on contrast enhanced MR images in patients with prostatitis. The ADC values obtained in all groups decreased with the increase in diffusion gradients. The distribution of ADC values of normal prostate parenchyma, prostatitis and prostate cancer group at b 100, 600 and 1,000 s/mm2 gradients are illustrated on Table 1.

Full Table
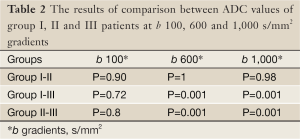
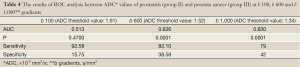
Mean ADC value of prostate cancer group (group III) was significantly lower than normal prostate parenchyma group (group I) (P=0.001) and prostatitis group (group II) (P=0.001) at b 600 and 1,000 s/mm2 gradients (Figures 1-6). No significant difference was obtained between ADC values of group III patients and group I and group II patients at b 100 s/mm2 gradient (P=0.72 and P=0.8, respectively). Mean ADC values of group I and II patients were not significantly different at b 100, 600 and 1,000 s/mm2 gradients (P=0.90, 1 and 0.98, respectively) (Table 2).
Full Table
The results of ROC analysis between ADC values of group I-III and group II-III patients are summarized in Tables 3,4, respectively. High sensitivity and low specificity values obtained in ROC analysis of ADC values in differentiation between group I-III patients and group II-III patients.

Full Table
Full Table
Discussion
The diagnosis of prostate cancer has been mainly based on TRUS guided biopsy. However TRUS guided biopsy is reported to have 40% false negative rates (12,13). Prostate cancer detection can be improved by imaging prostate with high- resolution T2-weighted scans and dynamic contrast enhanced MRI examination.
DWMRI is an emerging imaging technique that is able to demonstrate signal alterations secondary to restriction of molecular water movement in biological tissues. The ADC value as a quantitative parameter of DWMRI represents the magnitude of molecular movement in biological tissues. The restriction of diffusion results in decreased ADC values on ADC maps generated from DW images. Since prostate cancer manifests with increased cellularity and altered glandular structure of prostate gland at histopathological examination the utility of DWMRI in the diagnosis of prostate cancer has been investigated before in several studies (8,9,14). These studies yielded significant difference between ADC values of prostate cancer and benign prostate lesions. The ADC values of cancerous lesions have been found lower than normal parenchyma of prostate (9,15). The sensitivity and specificity of DWMRI for prostate cancer detection were reported as 57-93.3% and 57-100%, respectively (16). The results of our study are in concordance with these results since we found significant difference between ADC values of normal prostate parenchyma and prostate cancer at b 600 and 1,000 s/mm2 gradients. However we found no significant difference between ADC values of prostate cancer and normal prostate parenchyma at b 100 s/mm2 gradient. The ADC values obtained at low diffusion gradients represent either molecular diffusion and perfusion characteristics of biological tissues. Blood perfusion cause increased ADC values even in the setting of diffusion restriction in the tissue at low diffusion gradients. The absence of significant difference between ADC values of prostate cancer and normal prostate parenchyma at b 100 s/mm2 gradient may be attributed to perfusion effect of blood flow in the prostate. Koo et al. investigated the sensitivity results of various diffusion gradients (b 300, 700, 1,000 and 2,000 s/mm2) in predicting prostate cancer localizations at 3 T MRI and they found that b 1,000 s/mm2 gradient revealed higher sensitivity values (85%) than other diffusion gradients (17).
Prostatitis may present as acute or chronic illness of prostate. Although presenting symptoms of acute and chronic prostatitis are different they may mimic prostate cancer on conventional MRI with the appearance of low signal intensity on T2-weighted images and early enhancement on contrast enhanced MR images. Differentiation between ADC values of normal prostate parenchyma and prostatitis could not be achieved in our study. This may be secondary to inadequate restriction of diffusion in prostatitis. Comparison of ADC values between prostatitis group (group II) and prostate cancer group (group III) yielded significant difference at b 600 and 1,000 s/mm2 gradients. Absence of difference between ADC values of group II and group III patients at b 100 s/mm2 gradient may also be attributed to increased ADC values of group III patients secondary to perfusion effect of blood flow. Prostatitis is characterized by increased cellularity consisted of inflammatory cells which may result in diffusion restriction. However, increased perfusion and extracellular fluid resulting from edema in prostatitis may increase ADC values. As a result, we could not achieve to obtain significant difference between ADC values of normal prostate parenchyma and prostatitis group in our study. The ADC values of prostate cancer, normal prostate tissue and prostatitis were also compared with 3T MRI studies which revealed significantly lower ADC values in prostate cancer than normal prostate tissue and prostatitis (18).
The results of our study revealed that b 600 and 1,000 s/mm2 gradients were more helpful in differentiation between ADC values of prostate cancers and normal prostate parenchyma. None of three diffusion gradients yielded significant difference in differentiation of normal prostate and prostatitis in all levels of prostate. In our study we found that increased b values representing increased strength of diffusion gradient resulted in decreased sensitivity and increased specificity in differentiation prostate cancer from normal prostate gland and prostatitis (Tables 3,4).
This study has some limitations. The main limitation of our study was high possibility of mismatch of DWMRI and histopathologic slices. Shriveling and deformation of prostate specimens after formaldehyde fixation and leaned position of prostate resulting in nonvertical prostate position by urethra on DW images are the major causes of this mismatch (19). This mismatch limits the optimal evaluation of correlation between DW images and histopathological slices. Although b 600 and b 1,000 gradients were helpful in differentiation prostate cancer from normal prostate and prostatitis in our study, ultra-high b values such as b 2,000 was reported to improve the diagnostic performance of MRI with high sensitivity and specificity values (20). We did not measure ADC values of prostate lesions and normal prostate parenchyma at ultra-high b values due to decreased signal to noise ratio at ultra-high b values on our 1.5 T MRI system. We also did not assess the signal intensity changes in prostate cancer and prostatitis which would be helpful in detection of these lesions. The sensitivity of ADC values in detection of prostate cancer were in concordance with the literature but specificity values were lower than previous reports (Table 3) (16). This was attributed to low patient numbers and lack of assessment of signal intensity changes on DW images in our study.
In conclusion, DWMRI with ADC measurement may be used as a complementary imaging method in differentiation of prostate cancer from normal prostate parenchyma and prostatitis at intermediate and high level diffusion gradients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II: individual countries. BJU Int 2002;90:174-84. [PubMed]
- Cova M, Squillaci E, Stacul F, et al. Diffusion-weighted MRI in the evaluation of renal lesions: preliminary results. Br J Radiol 2004;77:851-7. [PubMed]
- Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497-505. [PubMed]
- Miller FH, Hammond N, Siddiqi AJ, et al. Utility of diffusion-weighted MRI in distinguishing benign and malignant hepatic lesions. J Magn Reson Imaging 2010;32:138-47. [PubMed]
- Parikh T, Drew SJ, Lee VS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology 2008;246:812-22. [PubMed]
- Doğanay S, Kocakoç E, Ciçekçi M, et al. Ability and utility of diffusion-weighted MRI with different b values in the evaluation of benign and malignant renal lesions. Clin Radiol 2011;66:420-5. [PubMed]
- Onur MR, Ozturk F, Aygun C, et al. Role of the apparent diffusion coefficient in the differential diagnosis of gastric wall thickening. J Magn Reson Imaging 2012;36:672-7. [PubMed]
- Issa B. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reson Imaging 2002;16:196-200. [PubMed]
- Sato C, Naganawa S, Nakamura T, et al. Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging 2005;21:258-62. [PubMed]
- Tanimoto A, Nakashima J, Kohno H, et al. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging 2007;25:146-52. [PubMed]
- Verma S, Rajesh A. A clinically relevant approach to imaging prostate cancer: review. AJR Am J Roentgenol 2011;196:S1-10 Quiz S11-4.
- Levine MA, Ittman M, Melamed J, et al. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol 1998;159:471-5. [PubMed]
- Svetec D, McCabe K, Peretsman S, et al. Prostate rebiopsy is a poor surrogate of treatment efficacy in localized prostate cancer. J Urol 1998;159:1606-8, discussion 475-6. [PubMed]
- Shimofusa R, Fujimoto H, Akamata H, et al. Diffusion-weighted imaging of prostate cancer. J Comput Assist Tomogr 2005;29:149-53. [PubMed]
- Van As N, Charles-Edwards E, Jackson A, et al. Correlation of diffusion-weighted MRI with whole mount radical prostatectomy specimens. Br J Radiol 2008;81:456-62. [PubMed]
- Turkbey B, Albert PS, Kurdziel K, et al. Imaging localized prostate cancer: current approaches and new developments. AJR Am J Roentgenol 2009;192:1471-80. [PubMed]
- Koo JH, Kim CK, Choi D, et al. Diffusion-weighted magnetic resonance imaging for the evaluation of prostate cancer: optimal B value at 3T. Korean J Radiol 2013;14:61-9. [PubMed]
- Nagel KN, Schouten MG, Hambrock T, et al. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology 2013;267:164-72. [PubMed]
- Yoshimitsu K, Kiyoshima K, Irie H, et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging 2008;27:132-9. [PubMed]
- Katahira K, Takahara T, Kwee TC, et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol 2011;21:188-96. [PubMed]