
Hepatic iron overload identified by magnetic resonance imaging-based T2* is a predictor of non-diagnostic elastography
Introduction
Systemic iron overload is a disorder characterized by high levels of plasma iron with subsequent deposition in multiple organs such as liver, pancreas, heart, pituitary gland and joints (1-3). Excess accumulation of iron may lead to life-threatening complications primarily related to the liver, including liver fibrosis, cirrhosis, liver failure and hepatocellular carcinoma (4,5). Thus, assessing for iron overload burden and monitoring for liver complications has been described in the management guidelines of various iron overload conditions such as hereditary hemochromatosis and thalassemia (6,7). Hepatic iron concentration provides a reliable indication of total body iron levels (6,8). The gold standard for measuring hepatic iron concentration is liver biopsy; however, liver biopsy is invasive, susceptible to sample variability from uneven hepatic iron concentration, and in rare cases confounded by severe and potentially life-threatening complications (9,10). Non-invasive magnetic resonance imaging, has recently been favored as an initial diagnostic test for assessing iron overload via the parameter T2*, an exponential decay constant that can be calculated from a single gradient-echo sequence with multiple echo times. T2* sequences have demonstrated a high sensitivity in detecting hepatic as well as cardiac iron deposition, even in the early stages of the disease (11-15), and T2* values have been shown to correlate well with biopsy-proven liver iron concentrations (16,17). Unlike the small samples obtained from liver biopsy, T2* includes data from a large cross-section of the liver.
Liver fibrosis is another important factor in predicting the prognosis and influencing the treatment of iron overload conditions (18). Magnetic resonance elastography (MRE) is a non-invasive imaging modality that measures liver stiffness and reliably determines the degree of liver fibrosis (19). Liver stiffness may occur in iron overload diseases due to iron deposition in the tissue, triggering the development of fibrosis, which in turn increases liver stiffness. Also, iron deposition by itself may directly contribute to increased liver stiffness.
Traditionally, MRE is a gradient echo technique. One of its main limitations is that susceptibility artifact from excess iron deposition degrades signal intensity in MRE sequences, leading to technical failure of elastography, resulting in non-diagnostic readings and decreasing MRE’s diagnostic reliability (20-22). As a result, patients with non-diagnostic MRE studies will not benefit from MRE in monitoring their disease, and will require alternative approaches, possibly including invasive liver biopsies.
To the best of our knowledge, there are no previous studies assessing the hepatic iron levels at which traditional MRE becomes non-diagnostic. In our study, we used T2* values to evaluate for hepatic iron overload and aimed to determine a cutoff T2* value above which MRE could provide accurate stiffness measurements. In addition, we assessed the ability of T2* values to predict the occurrence of non-diagnostic elastography in iron overload patients.
Methods
Study subjects
A total of 95 consecutive iron overload patients who underwent MRE at the Mayo Clinic in Jacksonville, Florida (MCF) between June 2010 and June 2017 were included in this retrospective study. Patients were extracted from an internal HIPAA compliant, IRB-approved database housing all patients seen at MCF with either: (I) an ICD9 diagnosis of iron overload or hemochromatosis; (II) a positive HFE gene mutations; (III) presenting to the Hereditary Hemochromatosis Clinic at MCF. MRE sequences were performed on 1.5 T scanners as previously described (Siemens Avanto, Berlin, Germany) (21). Briefly, a plastic drum driver is held in place against the anterior body wall by an elastic band. MRE two-dimensional gradient-echo sequences were then applied through 4 sections of the liver, each during a single breath-hold while 60 Hz acoustic pressure waves are applied to the plastic driver. Liver stiffness confidence maps were generated via an established post-processing technique (21), and stiffness measured manually from a given region of interest. The average of the liver stiffness measurements form the 4 slices yielded the recoded liver stiffness. Other MRI parameters were obtained concurrently on one of several 1.5T scanners at our institution (Siemens Avanto, Espree, or Aera). Liver T2* values were generated by methods previously reported (14,17). Briefly, a transverse slice through the liver was obtained during a single breath-hold multi-echo gradient echo sequence with 12 echo times ranging between approximately 1 and 22 ms, depending on the scanner. From these images a T2* map of the imaged liver slice are generated, with T2* measured manually from a given region of interest. All patients in the database who had undergone MRE were reviewed. Information was collected regarding patient characteristics [age, sex, weight, body mass index (BMI), race, hereditary hemochromatosis, diabetes, chronic kidney disease, anemia, history of iron overload, family history of iron overload, and history of alcohol use] and liver MRI information (HCC, iron overload, hepatic steatosis, cirrhotic morphology, MRE value, liver lesion, fat, and T2*). The primary outcome measure of the study was occurrence of a non-diagnostic elastography.
Statistical analysis
Continuous variables were summarized with the sample median and range. Categorical variables were summarized with number and percentage of patients. Comparisons of T2* values as well as other patient and MRI characteristics between patients with adequate and non-diagnostic elastography were made using a Wilcoxon rank sum test or Fisher’s exact test. We examined the ability of T2* measurements to predict occurrence of non-diagnostic elastography by estimating area under the ROC curve (AUC); an AUC of 0.5 indicates predictive ability equal to chance and an AUC of 1.0 indicates perfect predictive ability. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were estimated. P values of 0.05 or lower were considered to be statistically significant. All statistical tests were two-sided. Statistical analyses were performed using SAS (version 9.4; SAS Institute, Inc., Cary, North Carolina).
Results
Patient and liver MRI characteristics
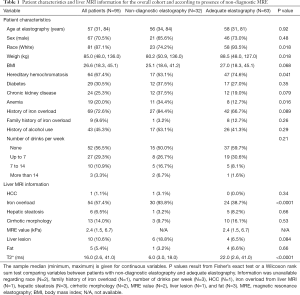
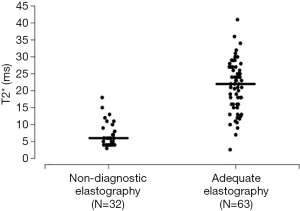
A summary of patient and liver MRI characteristics is shown in Table 1 for the overall cohort of 95 patients as well as separately for patients with non-diagnostic elastography (N=32, 33.7%) and those with adequate elastography (N=63, 66.3%). Patient characteristics that differed significantly between patients with and without a non-diagnostic elastography included race (White: 74.2% vs. 93.5%, P=0.018), weight (median: 80.2 vs. 88.5 kg, P=0.018), hereditary hemochromatosis (53.1% vs. 74.6%, P=0.041), and anemia (34.4% vs. 12.7%, P=0.016). For liver MRI information, significant differences between the non-diagnostic and adequate elastography groups occurred for iron overload (93.8% vs. 38.7%, P<0.0001) and the primary measure of the interest in the study, T2* (median: 6.0 vs. 22.0 ms, P<0.0001).
Full table
Prediction of non-diagnostic elastography by T2* scores
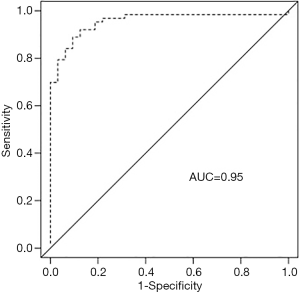
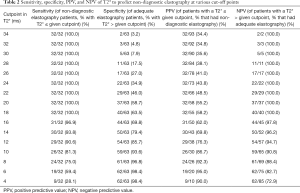
T2* predicted non-diagnostic elastography with an AUC of 0.95 at a cutoff of 20 ms, and this is shown in further detail in Figures 1 and 2. Sensitivity, specificity, PPV, and NPV estimates are shown in Table 2. All 32 patients with non-diagnostic elastography had a T2* value below 20 ms, and correspondingly of the 58 patients with a T2* value of 20 ms or lower, 32 (55%) had non-diagnostic elastography. The subgroups of patients with a T2* value ≤10 ms (N=30), ≤8 ms (N=26), and ≤6 ms (N=20), had a particularly high likelihood of non-diagnostic elastography (87%, 92%, and 95%, respectively).
Full table
Discussion
Previous studies have described various factors associated with technical failure of MRE, with iron overload being one of them, but no further sub-analysis was performed in this particular subset of patients (20,21). In this study, we focused on evaluating a group of patients diagnosed with iron overload conditions who had non-diagnostic MRE results.
In our study, the presence of anemia and a diagnosis of hereditary hemochromatosis were associated with non-diagnostic elastography exams. We did not perform further analysis of these findings but realize that patients with anemia may receive more frequent blood transfusions, which may represent severe hematological disease that eventually resulted in higher total body and hepatic iron concentrations. Conflicting data has been reported in regards to effects of BMI on technical accuracy of MRE. In a study by Wagner et al. (20), BMI was independently associated with increased technical failure rates of MRE with a low odds ratio of 1.09. On the contrary, studies by Yin et al. (21) and Chen et al. (23) showed no statistical difference in BMI between successful and failed MRE examinations. In our study, the non-diagnostic and adequate elastography groups were both in the overweight category based on the median BMI (25.1 and 27.0 kg/m2 respectively, P=0.068) with no statistical difference. We have noticed a statistically significant trend between the weights of the non-diagnostic and adequate elastography groups (80.2 and 88.5 kg, P=0.018) which is difficult to analyze without further available information such as weight circumference or subcutaneous fat thickness. As expected and previously described in literature (21,24), our study suggests that the presence of hepatic iron overload on MRI readings was more frequently associated with non-diagnostic MRE results. In addition, the T2* values were significantly lower in patients with non-diagnostic elastography when compared to patients with adequate elastography (median: 6.0 vs. 22.0 ms, P<0.0001). This is consistent with the known signal degradation caused by increased iron in tissue when using gradient echo-based MRI sequences (25). Newer elastography sequences derived from spin-echo techniques show promise in quantifying liver fibrosis even in the presence of hepatic iron overload (26); however, the traditional gradient echo technique is more prevalent in routine clinical practice.
In our patient cohort, a hepatic T2* cut-off value of 20 ms (at 1.5 T) was predictive for non-diagnostic elastography with an AUC of 0.95. For clinical relevance, a value of T2* 20 ms correlated to liver iron concentration (LIC) of 1.5 mg/g dry tissue based on a previously published conversion formula (17). Despite the variant literature, the 95% upper limit of normal LIC is mostly described to be around 1.8-mg/g dry tissue (27). A study by Bassett et al. suggested that the cut-off value for LIC at which histological evidence of hepatic damage occurs in patents with genetic hemochromatosis homozygotes was 22.3 mg/g dry weight (28). Our subgroups of patients with a T2* value ≤10 ms equivalent to LIC of ≥2.7 mg/g, ≤8 ms equivalent to LIC of ≥3.6 mg/g, and ≤6 ms equivalent to LIC of ≥5.1 mg/g, had a particularly high likelihood of a non-diagnostic elastography (87%, 92%, and 95%, respectively).
Our findings suggest that T2* values can accurately predict non-diagnostic elastography outcomes. Even with a “normal” LIC level of 1.5 mg/g, 55% of the MRE readings will be non-diagnostic. The higher the LIC values, the higher likelihood of non-diagnostic elastography results with a 95% likelihood with LIC levels ≥5.1 mg/g. It may therefore be necessary to resort back to liver biopsy to assess fibrosis in such patients until spin-echo MRE techniques become better validated and more widespread.
Several limitations of this study are important to bear in mind. First, the retrospective nature of our study may have negatively affected the accuracy of data collection. Second, the number of patients with non-diagnostic elastography was relatively small, and therefore validation of these findings of a larger series of iron overload patients will be important. Third, the MR parameters measured were all performed on 1.5 T scanners. T2* values at 1.5 and 3 T are related (T2* at 1.5 T is roughly double that at 3 T), but they are not identical (29). Therefore, our numerical results for T2* should be extrapolated to 3 T systems with caution. Additionally, at extremely high liver iron concentrations, rapid signal decay during the gradient echo sequence makes precise iron concentration measurements challenging, and this is exacerbated in higher field strength scanners (15).
In conclusion, the results of this study suggest that T2* values may accurately predict which patients will have a non-diagnostic MRE exam. Specifically, while a T2* of 20 ms or lower (on a 1.5 T scanner) may signal the possibility of non-diagnostic elastography, values of ≤10, ≤8, and ≤6 ms appear to indicate an especially high risk. Conversely, if T2* is greater than 20, the likelihood of non-diagnostic elastography appears very low as evidenced by the lack of any non-diagnostic elastography patients with a T2* value greater than 20 ms in our study.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med 2012;366:348-59. [Crossref] [PubMed]
- Brissot P, Loreal O. Iron metabolism and related genetic diseases: A cleared land, keeping mysteries. J Hepatol 2016;64:505-15. [Crossref] [PubMed]
- Finch SC, Finch CA. Idiopathic hemochromatosis, an iron storage disease. A. Iron metabolism in hemochromatosis. Medicine (Baltimore) 1955;34:381-430. [Crossref] [PubMed]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004;127:S79-86. [Crossref] [PubMed]
- Willis G, Wimperis JZ, Lonsdale R, Fellows IW, Watson MA, Skipper LM, Jennings BA. Incidence of liver disease in people with HFE mutations. Gut 2000;46:401-4. [Crossref] [PubMed]
- Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. American Association for the Study of Liver D. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:328-43. [Crossref] [PubMed]
- Mancuso A. Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol 2010;2:171-4. [Crossref] [PubMed]
- Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 2000;343:327-31. [Crossref] [PubMed]
- Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL, Dienstag JL. Group H-CT. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:877-83. [Crossref] [PubMed]
- Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol 1999;5:301-4. [Crossref] [PubMed]
- Sarigianni M, Liakos A, Vlachaki E, Paschos P, Athanasiadou E, Montori VM, Murad MH, Tsapas A. Accuracy of magnetic resonance imaging in diagnosis of liver iron overload: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2015;13:55-63.e5. [Crossref] [PubMed]
- Assis RA, Kay FU, Conti FM, Campregher PV, Szarf G, Diniz MS, Rodrigues M, Helman R, Funari MB, Wood J, Hamerschlak N. The role of magnetic resonance imaging-T2* in the evaluation of iron overload early in hereditary hemochromatosis. A cross-sectional study with 159 patients. Am J Hematol 2015;90:E220-1. [Crossref] [PubMed]
- Zamani F, Razmjou S, Akhlaghpoor S, Eslami SM, Azarkeivan A, Amiri A. T2* magnetic resonance imaging of the liver in thalassemic patients in Iran. World J Gastroenterol 2011;17:522-5. [Crossref] [PubMed]
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001;22:2171-9. [Crossref] [PubMed]
- Hernando D, Levin YS, Sirlin CB, Reeder SB. Quantification of liver iron with MRI: state of the art and remaining challenges. J Magn Reson Imaging 2014;40:1003-21. [Crossref] [PubMed]
- Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005;106:1460-5. [Crossref] [PubMed]
- Garbowski MW, Carpenter JP, Smith G, Roughton M, Alam MH, He T, Pennell DJ, Porter JB. Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan. J Cardiovasc Magn Reson 2014;16:40. [Crossref] [PubMed]
- Beaton MD, Adams PC. Prognostic factors and survival in patients with hereditary hemochromatosis and cirrhosis. Can J Gastroenterol 2006;20:257-60. [Crossref] [PubMed]
- Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135:32-40. [Crossref] [PubMed]
- Wagner M, Corcuera-Solano I, Lo G, Esses S, Liao J, Besa C, Chen N, Abraham G, Fung M, Babb JS, Ehman RL, Taouli B. Technical Failure of MR Elastography Examinations of the Liver: Experience from a Large Single-Center Study. Radiology 2017;284:401-12. [Crossref] [PubMed]
- Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL, Hepatic MR. Elastography: Clinical Performance in a Series of 1377 Consecutive Examinations. Radiology 2016;278:114-24. [Crossref] [PubMed]
- Bota S, Sporea I, Sirli R, Popescu A, Danila M, Jurchis A, Gradinaru-Tascau O. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography--analysis of a cohort of 1,031 subjects. Eur J Radiol 2014;83:268-72. [Crossref] [PubMed]
- Chen J, Yin M, Talwalkar JA, Oudry J, Glaser KJ, Smyrk TC, Miette V, Sandrin L, Ehman RL. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology 2017;283:418-28. [Crossref] [PubMed]
- Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, Lee KB, Han JK, Choi BI. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology 2014;273:772-82. [Crossref] [PubMed]
- Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544-55. [Crossref] [PubMed]
- Mariappan YK, Dzyubak B, Glaser KJ, Venkatesh SK, Sirlin CB, Hooker J, McGee KP, Ehman RL. Application of Modified Spin-Echo-based Sequences for Hepatic MR Elastography: Evaluation, Comparison with the Conventional Gradient-Echo Sequence, and Preliminary Clinical Experience. Radiology 2017;282:390-8. [Crossref] [PubMed]
- St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005;105:855-61. [Crossref] [PubMed]
- Bassett ML, Halliday JW, Powell LW. Value of hepatic iron measurements in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology 1986;6:24-9. [Crossref] [PubMed]
- Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J Magn Reson Imaging 2007;25:540-7. [Crossref] [PubMed]


