
Primitive neuroectodermal tumors: a clinical and radiological analysis of six cases
Introduction
Primitive neuroectodermal tumor (PNET) is a kind of extremely rare and highly aggressive, small round cell tumor, with poor prognosis. It develops from the primitive nerve cells of the nervous system (1). It is a member of Ewing’s sarcoma family, mainly occurring in adolescents and in children (2). According to the differential classification, it can be divided into peripheral PNET (pPNET) and central PNET (cPNET). The cPNETs arise from a precursor cell of the subependymal matrix of the central nervous system (CNS) or external granular layer of the cerebellum, pinealocytes, and subependymal cells of the ventricles whereas pPNETs derive from the neural crest located outside the CNS (1). pPNET is the most common, it occurs most in the chest wall, followed by the pelvis, retroperitoneum, abdomen and neck in turn (3). PNET has characteristics of low incidence, high degree of malignancy, rapid progression, high rate of recurrence and metastasis with a poor prognosis (4). Although the clinical and pathological characteristics of PNET have been reported, few studies concerning the imaging features have been published (5). We report six patients with PNET in this paper.
Methods
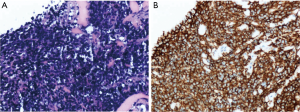
This retrospective study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of China Medical University. Informed consent was waived due to the retrospective and non-interventional nature of this analysis. Six patients with pathologically confirmed PNET were treated in the First Affiliated Hospital of China Medical University, Shenyang, China, during Jan 2012 to Dec 2016. The patients included three males and three females, ranging in age from 20 to 48 years old. The main clinical manifestation was symptoms caused by rapid-growing mass. Pathology and immunohistochemistry confirmed the tumor final diagnosis were PNETs. Pathological findings presented that heterogeneous cells were distributed in flaky nest-like shape, arranged densely, with hyperchromatic nuclei, bare nucleus and significantly atypia (Figure 1A). By immunohistochemistry, the high expression of CD99 in tumor tissues further supported the final diagnosis of PNETs (Figure 1B). All the six cases had tumor cells positive for CD99. The general information of these six cases is listed in Table 1. One case (case 4) had undergone only radiotherapy and not surgery because of her physical condition.
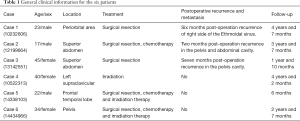
Full table
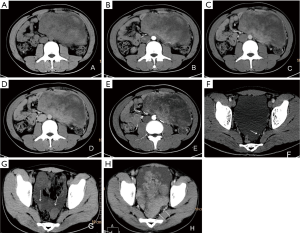
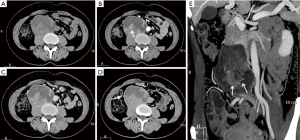
Six patients were followed up, with a mean follow-up period of 34.5 months (ranging from 6 to 55 months). Five patients survived and one died. Among the five patients undergoing surgeries, one patient presented pelvic and abdominal recurrence/metastasis 2 months after abdominal PNET resection (Figure 2). One patient had a recurrent lesion in the right orbit involving the right ethmoid sinus 6 months after right orbital PNET resection. One patient’s pelvic tumor recurred 7 months after PNET operation, and this patient died after 1 year and 10 months of follow-up. During the follow-up period, the remaining three cases did not show obvious recurrence and/or metastasis.
Computed tomography (CT) examinations (cases 2–6) used Toshiba CT scanning system (Aquilion ONE, Japan). Scan parameters: spiral scan mode, thickness 0.5 mm, matrix 1,024×1,024. magnetic resonance (MR) examinations (cases 1 and 5) used 3.0T MR equipment (GE Signa Advantage Hdxt, USA).
Results
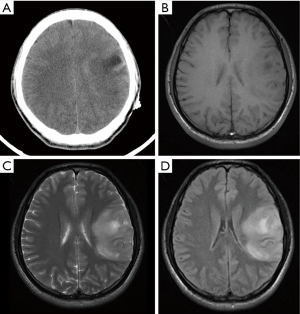
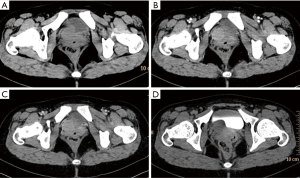
PNET cases’ imaging findings are presented in Table 2 and Figures 2-5. The tumor’s density was uniform for small tumor and heterogeneous for large tumors on CT images, while the size of tumors differed during presentation depending on the location of the tumor. Marked enhancement was visualized after injection of contrast media. The demarcation between the lesion and adjacent tissues or organs tended to be unclear (Figures 3,5). Tortuous blood vessels within the masses could be observed after enhancement (Figures 2E,3E). On magnetic resonance imaging (MRI) images, the mass mainly showed heterogeneously long T1 and long T2 signal intensity, mixed high signal intensity on fluid-attenuated inversion recovery (FLAIR) image (Figure 4B,C,D).
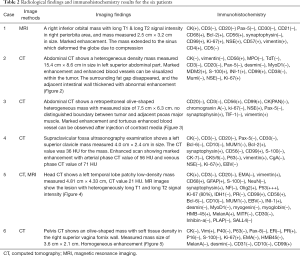
Full table
Discussion
PNET is a rare and highly malignant tumor arising from the neural crest that was. It is a member of Ewing’s sarcoma family (1), often occur in youth and children. PNETs can be divided into cPNET, that develops from the CNS, most commonly the cerebellum, and pPNET arising from the neural crest. Both are aggressive tumors and have similar survival rates (2). Both types have characteristics of a high degree of malignancy, a common distant metastasis, and local recurrence. The incidence ratio of male to female is around 3:1 (5,6). PNET tends to recur locally and to metastasize early to regional lymph nodes, lungs, liver, bone, and bone marrow within 2 to 3 years after surgery, with a resulting of very poor prognosis.
PNET patients usually have no specific symptoms/signs on physical examination or no special presentations on imaging exams and serum biochemical tests. The clinical presentation of PNET is variable, and related to tumor location, size, and invasiveness. The tumors in our study are located in the orbit, temporal region, pelvic, and abdomen, which is consistent with previous reports (7). The diagnosis of PNET is dependent on pathological examination and immunohistochemistry. Microscopically, PNET is mainly composed of small round cells, which are diffusively distributed or form several lobulated structures. In addition, tumor cells may be positive for other proteins related to neural differentiation, including neuron-specific enolase (NSE), S-100 protein, neurofilament, synaptophysin, and chromogranin A. NSE, CD99 and vimentin positive can help confirm the diagnosis. In addition, chromosome ectopic (11; 22) (q24; q12) is highly specific and it can have additional diagnostic standards (7-10).
There is no current consensus regarding the optimal treatment protocol for PNET (11). The cornerstone of therapy has been surgical resection of the tumor (12,13). Due to the scarcity of cases of this disease, the standard first-line adjuvant treatment remains unclear. There is no consensus on the chemotherapy regimens in the patients with PNET; however, there are reports recommending cyclophosphamide or ifosfamide, cisplatin or carboplatin and VP-16 (etoposide) (14). In recent years, some literature reported that chemotherapy using sunitinib, sorafenib, and bevacizumab might be valuable in the treating PNET (8). Radiotherapy is believed to be an important adjuvant treatment option for PNET. Our study tentatively supports the effectiveness of radiotherapy as an adjuvant treatment option for PNET (Table 1).
Previous studies (15) reported that PNET mostly shows mixed isointense to hypointense signals on T1-weighted imaging, and isointense to hyperintense signals on T2-weighted imaging. PNET may have heterogeneous enhancement on CT scan and significant enhancement on the MRI (16). It can be seen from our cases that the tumor density may appear uniform when it is small and the tumor may appear heterogeneous when it is large in size. PNETs generally do not have clear boundary with its adjacent organs or tissues suggesting their invasive nature. It may be a diagnostic valuable sign when maximum intensity projection reconstruction image demonstrates tortuous blood vessels within the tumor on enhanced CT images.
In conclusion, the clinics and imaging manifestations of six cases of PNET are presented in this report. Generally, the imaging appearances of PNET lack characteristics. When the tumor is small its density may appear uniform and it tends to become heterogeneous when the tumor is large. PNETs generally do not have clear boundary, or partially so, with its adjacent organs or tissues suggesting their invasive nature. Upon further validation, maximum intensity projection image reconstruction demonstrates tortuous blood vessels within the tumor on enhanced CT images may be valuable information for diagnosis of PNET.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of China Medical University. Informed consent was waivered due to the retrospective and non-interventional nature of this analysis.
References
- Dai J, He HC, Huang X, Sun FK, Zhu Y, Xu DF. Long-term survival of a patient with a large adrenal primitive neuroectodermal tumor: A case report. World J Clin Cases 2019;7:340-6. [Crossref] [PubMed]
- Nery B, Pereira LCT, Costa RAF, Queiroz RM, Abud LG, Quaggio E, Coronatto LH, Prado IST, Miyake CH, Filho FB. Cervicomedullary primitive neuroectodermal tumor of the spine: Case report. Surg Neurol Int 2018;9:241. [Crossref] [PubMed]
- Rahbar M, Rahbar M, Bahoush G. Peripheral primitive neuroectodermal tumor associated with paraneoplastic Cushing's syndrome: The rare case. Ann Med Surg (Lond) 2018;37:21-4. [Crossref] [PubMed]
- Ma J, Ma S, Yang J, Jia G, Jia W. Primary spinal primitive neuroectodermal tumor: A single center series with literature review. J Spinal Cord Med 2018.1-9. Epub ahead of print. [Crossref] [PubMed]
- Aguiar TF, Barbosa-Teixeira AC, Costa SS, Ezquina S, Gimenez TM, Novak E, Cristofani LM, Rosenberg C, Odone Filho V, Krepischi ACV. Atypical presentation of a germline APC mutation in a child with supratentorial primitive neuroectodermal tumor. Pediatr Blood Cancer 2019;66:e27566. [Crossref] [PubMed]
- Abolhasani M, Salarinejad S, Moslemi MK. Ewing sarcoma/primitive neuroectodermal tumor of the kidney: A report of three cases. Int J Surg Case Rep 2016;28:330-4. [Crossref] [PubMed]
- Chen J, Jiang Q, Zhang Y, Yu Y, Zheng Y, Chen J, Zhao Y, Miao Z, Fan F, Wang Y. Clinical Features and Long-Term Outcome of Primary Intracranial Ewing Sarcoma/Peripheral Primitive Neuroectodermal Tumors: 14 Cases From a Single Institution. World Neurosurg 2019;122:e1606-14. [Crossref] [PubMed]
- Thomas AC, Rajashekharan R. A Rare Case of Dumbbell-shaped Primary Intraspinal Peripheral Primitive Neuroectodermal Tumor Involving Thoracic Spinal Epidural Space. Asian J Neurosurg 2018;13:1216-8. [Crossref] [PubMed]
- Chen J, Yuan T, Liu X, Hua B, Dong C, Liu Y, Quan G. Ewing's Sarcoma/Peripheral Primitive Neuroectodermal Tumors in Bronchus. Am J Med Sci 2019;357:75-80. [Crossref] [PubMed]
- Zhang C, Zhang J, Wang G, Xu J, Li Y, Guo Q, Zheng T, Zhang Y. Benefit of Sunitinib in the treatment of pulmonary primitive neuroectodermal tumors: a case report and literature review. Oncotarget 2016;7:87543-51. [Crossref] [PubMed]
- Jaramillo-Huff A, Bakkar R, McKee JQ, Sokkary N. Primary Primitive Neuroectodermal Tumor Arising from an Ovarian Mature Cystic Teratoma in a 12-Year-Old Girl: A Case Report. J Pediatr Adolesc Gynecol 2017;30:511-2. [Crossref] [PubMed]
- Soles BS, Wilson A, Lucas DR, Heider A. Melanotic Neuroectodermal Tumor of Infancy. Arch Pathol Lab Med 2018;142:1358-63. [Crossref] [PubMed]
- Liu Z, Xu YH, Ge CL, Long J, Du RX, Guo KJ. Huge peripheral primitive neuroectodermal tumor of the small bowel mesentery at nonage: A case report and review of the literature. World J Clin Cases 2016;4:306-9. [Crossref] [PubMed]
- Sublett JM, Davenport C, Eisenbrock H, Dalal S, Jaffar Kazmi SA, Kershenovich A. Pediatric Primary Diffuse Leptomeningeal Primitive Neuroectodermal Tumor: A Case Report and Literature Review. Pediatr Neurosurg 2017;52:114-21. [Crossref] [PubMed]
- Cherif El Asri A, Benzagmout M, Chakour K, Chaoui MF, Laaguili J, Chahdi H, Gazzaz M, El Mostarchid B. Primary Intracranial pPNET/Ewing Sarcoma: Diagnosis, Management, and Prognostic Factors Dilemma-A Systematic Review of the Literature. World Neurosurg 2018;115:346-56. [Crossref] [PubMed]
- Baleato-González S, Tirapu-de-Sagrario MG, Pintos-Martínez E, García-Figueiras R. Scrotal Peripheral Primitive Neuroectodermal Tumor. Curr Urol 2018;12:50-3. [Crossref] [PubMed]


