
Metabolic parameters with different thresholds for evaluating tumor recurrence and their correlations with hematological parameters in locally advanced squamous cell cervical carcinoma: an observational 18F-FDG PET/CT study
Introduction
About one-third of locally advanced cervical carcinoma (LACC) patients routinely treated with concurrent chemoradiotherapy (CCRT) relapse within 18 months after treatment and have a poor prognosis (1). A timely and accurate prognosis assessment is important for such patients because their treatment plan can be tailored in a timely manner or more/less intensive follow-up can be arranged to improve these patients’ survival and quality of life. Traditional factors such as Federation International of Gynecology and Obstetrics (FIGO) stage, tumor size, histological characteristics, biological characteristics and lymphatic metastasis are significant prognostic factors for tumor recurrence and survival in cervical cancer (2-7). Lymphatic metastasis is the most generally recognized of these factors, whereas the value of other factors remains controversial (4-9). Therefore, many research efforts have been devoted to the discovery of more accurate predictors.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been widely used for preoperative management of cervical cancer. PET/computed tomography (CT) imaging facilitates the assessment of primary lesions and suspicious metastatic lymph nodes (SMLNs). Moreover, its semiquantitative parameters [maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG)] are also good prognostic markers for LACC (7-11). SUVmax of the primary tumor has been regarded as a reliable marker of prognosis in previous studies (9,11). MTV and TLG for the primary tumor, calculated by using a certain SUVmax threshold, were thought to be better indicators for patients with LACC (7,8,10). In addition to the status of the primary tumor, the status of lymph nodes (LNs) on PET was also proven to be very important for LACC prognosis (5-9). Certain researchers (12-14) have concluded that PET parameters for a combination of the primary tumor and SMLNs have a higher prognostic value than those for the primary tumor alone. Identifying the optimal SUVmax threshold for tumor recurrence evaluation appears to be particularly crucial, with most studies using an absolute threshold of SUV>2.5 or a relative threshold of >40% of SUVmax (6,7,11-13,15). Other thresholds could potentially be more appropriate for semiquantitative assessment of tumor recurrence, as some preliminary studies of primary lesions alone have suggested (16,17).
In addition to traditional prognostic factors, certain hematological parameters are also closely related to prognosis for LACC, including squamous cell carcinoma antigen (SCC-ag), systemic inflammatory response biomarker levels [mainly the neutrophil-lymphocyte ratio (NLR) and the platelet-lymphocyte ratio (PLR)], and hemoglobin (Hb) (2-4,18-20). Both PET-derived metabolic parameters and hematological parameters can assess prognosis, but it is unclear whether there are any correlations between these two types of parameters. If such correlations exist, PET parameters and hematological parameters may provide complementary information that could be combined for prognosis assessment. To the best of our knowledge, while several reports (21,22) have explored how PET parameters are correlated with NLR and PLR, no reports have addressed this topic for LACC. Therefore, this study was conducted for the following purposes: (I) to compare the values of SUVmax, MTV, and TLG of the primary tumor (SUVmax-P, MTV-P, and TLG-P, respectively) and the combination of the primary tumor and SMLNs (SUVmax-C, MTV-C, and TLG-C, respectively) based on different percentages of SUVmax (%SUVmax) thresholds for evaluating tumor recurrence and find the optimal PET parameter; (II) to investigate correlations between PET parameters and hematological parameters to determine the most relevant PET parameters and optimal thresholds; (III) and to explore independent risk factors affecting recurrence-free survival (RFS) for LACC.
Methods
Patients
Between August 2014 and September 2016, a total of 112 patients in our hospital with FIGO stage IIb-IVA squamous cell cervical carcinoma (SCCC) underwent 18F-FDG PET/CT for initial diagnosis and systemic assessment. These patients were prepared to receive CCRT as their treatment option. Patients with an age of less than 30 years or more than 80 years, other malignant tumors or other systemic diseases (such as cirrhosis, cardiovascular disease, and diabetes, among others) were excluded. Eventually, a total of 89 patients satisfied the criteria and were enrolled in the study. Informed consent was obtained from all patients.
All patients underwent biopsy, gynecological examination, cystoscopy, pelvic magnetic resonance imaging (MRI) and routine blood tests. Biopsy was used to determine tumor subtype and differentiation. Gynecological examination, cystoscopy and medical imaging examination could help identify FIGO stage and tumor size. SCC-ag, neutrophil count, platelet count, lymphocyte count and Hb were obtained via blood tests, with normal values of 0–1.5 ng/mL, (1.9–7.2)×109/L, (135–350)×109/L, (1.1–2.7)×109/L, and (110–150) g/L, respectively.
PET/CT imaging and analysis
Prior to undergoing PET/CT scans, all patients were required to fast for 4–6 hours and have a blood glucose level ≤11.1 mmol/L. Images were acquired on a PET/CT scanner (GE Discovery Elite; GE Healthcare, USA) at 64.2±8.3 min after the injection of 3.7–5.55 MBq/kg (0.1–0.15 mCi/kg) of 18F-FDG (MiniTrace II and TraceLab FXFDG; GE, USA; purity >99%). Low-dose CT with a tube voltage of 120 kV, a tube current of 80 mA, and a slice thickness of 3.27 mm was used. CT scans were performed from the top of the skull to midthigh (2 min/bed) and then for the head and neck (5 min/bed). Subsequently, PET data were acquired using a three-dimensional acquisition mode at a speed of 1.5 min/bed and with a matrix size of 192×192. The ordered-subsets expectation maximization iterative reconstruction algorithm (24 subsets, 2 iterations) and the time-of-flight and point-spread function techniques were used for attenuation correction of PET images. The PET/CT data were postprocessed on an Advantage Workstation 4.6 (GE Medical Systems, Milwaukee, WI, USA) using PET Volume Computerized Assisted Reporting (PET VCAR) software.
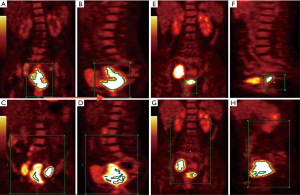
Measurements were conducted by two radiologists with 10 and 15 years of experience in nuclear medicine who were blinded to patients’ clinical and pathological results. When these two radiologists disagreed, they reached consensus via discussion. First, the two radiologists read the coronal, sagittal, and axial PET images and PET/CT fusion images of a patient. They could observe the FDG hypermetabolic lesion in the cervix and SMLNs in the pelvic and para-aortic regions for each patient (if present). On coronal and sagittal PET images of the whole body, the initial regions, represented by the green box in Figure 1, were placed in a range, including only primary tumors and excluding physiological FDG uptake (Figure 1A,B,E,F). The tumor contour, defined as the volume of interest (VOI), was delineated automatically to include voxels presenting SUV values greater than different %SUVmax thresholds (from 30%SUVmax to 60%SUVmax in increments of 5%) in the primary tumor. SUVmax-P (the highest SUV within the tumor VOI), MTV-P (the sum of the volumes of all voxels greater than a certain SUVmax threshold) and TLG-P (SUVmean multiplied by MTV) were calculated. Then, the radiologists expanded the green box to include the primary lesion and all SMLNs (if present) (Figure 1C,D,G,F). SUVmax-C, MTV-C, and TLG-C were acquired. When n%SUVmax was used as the threshold to define the tumor margins of the primary tumor or the whole lesion, the corresponding MTV values were denoted as MTV-Pn% and MTV-Cn%, and the corresponding TLG values were denoted as TLG-Pn% and TLG-Cn%.
CCRT
All enrolled patients completed CCRT consisting of five weeks of external beam radiotherapy (EBRT) at a dose of 1.8 Gy daily to achieve a total dose of 45 Gy, and intracavitary brachytherapy with a total dose of 30 Gy (6 Gy/fraction, 5 fractions). In addition, all patients received cisplatin-based (40 mg/d) chemotherapy weekly during the period of EBRT.
Clinical and imaging follow-ups
After patients had completed CCRT, they were followed up every 3 months for the first 2 years and every 6 months for the next 3 to 4 years (via biopsy or follow-up imaging). RFS referred to the time between CCRT completion and initial confirmation of progression and/or recurrence. The progression and/or recurrent event could have been local, nodal, and/or metastatic and could have been detected by clinical examination, MRI, and/or 18F-FDG PET/CT. When any progressive and/or recurrent disease was detected, the affected sites and time were recorded.
Statistical analysis
PET parameters and hematological parameters were analyzed as continuous variables and expressed as median (range). Harrell’s c-index (c-index) was calculated to evaluate the predictive capabilities of PET parameters for RFS (a c-index of 0.5 suggests that a parameter is not predictive, whereas a c-index of 1 signifies that a parameter is predictive) (17). The c-index is equal to the area under the curve (AUC) for a receiver operating characteristic (ROC) curve, which represents the tradeoff between a predictor’s sensitivity and specificity. A Cox regression model was used to perform univariate and multivariate analyses of prognostic significance of PET parameters estimated by using the optimal threshold. Survival curves were estimated and compared using the Kaplan-Meier method and log-rank tests. The median of the optimal PET parameters was used as the cutoff point for dichotomization. Spearman correlation analysis was utilized to analyze correlations between PET parameters and hematological parameters. Strength of agreement was evaluated by using correlation coefficients (<0.00 being poor; 0.00–0.40 being slight, 0.41–0.70 being moderate, and 0.71–1.00 being high). All statistical analyses was performed using R and IBM SPSS version 22.0 software.
Results
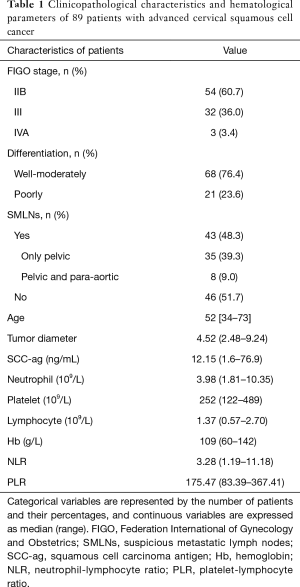
Clinicopathological features of enrolled patients are presented in Table 1. Overall, 43 of 89 (48.3%) patients showed SMLNs on PET, and 46 of 89 (51.7%) patients did not have SMLNs. After the follow-up period (mean 24.7 months, ranging from 4.5–51.0 months), 70 patients relapsed, 3 patients were lost to follow-up, and only 16 patients had no evidence of progression and/or recurrence. Local recurrence was found in 21 patients (including 5 patients with only local recurrence and 16 patients with local recurrence and metastasis), and LNs recurrence was detected in 33 patients (including 20 patients with recurrence in the pelvis or para-aortic area, and 13 patients with recurrence in the para-aortic area only). There were 16 patients with metastatic disease without local recurrence.
Full table
PET metabolic parameters based on different %SUVmax thresholds
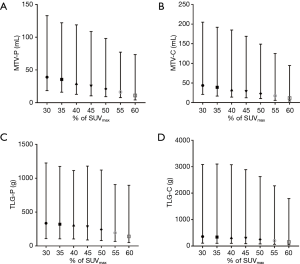
The median SUVmax-P and SUVmax-C values were 17.56 (range, 9.49–32.58) and 18.14 (range, 9.49–32.58), respectively. The median MTV-P and MTV-C values calculated by using the 50%SUVmax threshold were 21.11 mL (range, 9.21–98.14 mL) and 23.04 mL (range,, 10.26–149.00 mL), respectively. The median TLG-P and TLG-C values estimated by using the 50%SUVmax threshold were 243.80 g (range, 79.98–1,120.76 g) and 252.46 g (range, 79.98–2,625.38 g), respectively. Other PET parameters for the primary tumor and the combination of the primary tumor and SMLNs are displayed in Figure 2A,B,C,D.
RFS evaluation of %SUVmax threshold-based PET metabolic parameters
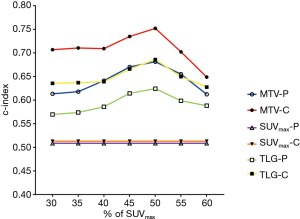
Figure 3 summarizes the c-index values for evaluating RFS for MTV and TLG determined by using different %SUVmax thresholds. All PET parameters had the highest c-index values with the 50%SUVmax threshold. With the same threshold, MTV-C and TLG-C had higher c-index values than MTV-P and TLG-P, respectively. The optimal PET parameter for RFS evaluation was MTV-C based on the 50%SUVmax threshold (c-index =0.752). Representative PET images of two patients with different RFS are presented in Figure 1.
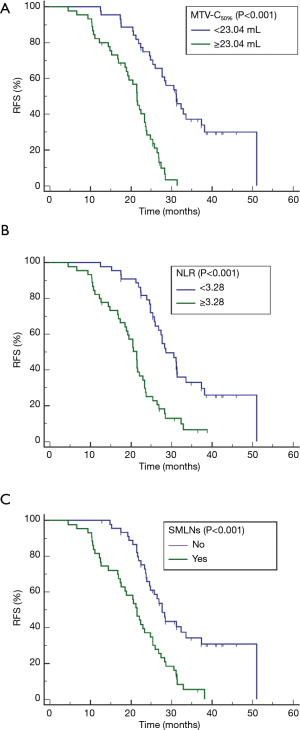
Univariate and multivariate analyses of prognostic factors for RFS are summarized in Table 2. Univariate analyses revealed that FIGO stage, tumor diameter, hematological parameters (SCC-ag, NLR, and PLR) and MTV-C50% were associated with RFS. Multivariate analysis indicated that MTV-C50% [hazard ratio (HR], 1.065; P<0.001], NLR (HR, 1.195; P=0.045) and SMLNs (HR, 2.225; P=0.003) were independent risk factors for tumor recurrence. Survival curves for MTV-C50% (cutoff value, 23.04 mL; P<0.001), NLR (cutoff value, 3.28; P<0.001), and SMLNs (yes vs. no; P<0.001) determined using the Kaplan-Meier method are shown in Figure 4.
Full table
Correlations between %SUVmax threshold-based PET metabolic parameters and hematological parameters
Tables 3 and 4 show correlation coefficients describing how SUVmax, MTV, and TLG were correlated with SCC-ag, NLR, PLR and Hb at different %SUVmax thresholds (from 30%SUVmax to 60%SUVmax in increments of 5%). SUVmax-P and SUVmax-C were not significantly correlated with any hematological parameters. With each %SUVmax threshold, MTV had a slight-to-moderate correlation with SCC-ag, NLR and PLR. TLG was correlated with SCC-ag, NLR and PLR with different %SUVmax thresholds, with the exception of not being correlated with PLR for certain %SUVmax thresholds. The correlation coefficients of MTV were greater than those of TLG with the same thresholds. The correlation coefficients of MTV-C and TLG-C were higher than those of MTV-P and TLG-P, respectively, with the same thresholds. PET parameters were not significantly related to Hb. For SCC-ag, MTV-P55% (r=0.408, P<0.001), TLG-P50% (r=0.353, P=0.001), MTV-C55% (r=0.500, P<0.001) and TLG-C55% (r=0.418, P<0.001) had the highest correlation coefficients among PET parameters for all tested thresholds. For NLR and PLR, MTV and TLG (r=0.637 and r=0.515, respectively; P<0.001 for both correlations) had the highest correlation coefficients with the 50%SUVmax threshold.

Full table

Full table
Discussion
This study explored the value of PET metabolic parameters for evaluating tumor recurrence, by testing different %SUVmax threshold segmentations, and evaluated correlations between these parameters and hematological parameters prior to treatment, including SCC-ag, systemic inflammatory response biomarkers (NLR and PLR) and Hb, for LACC treated with CCRT. Our results proved that MTV-C and a 50%SUVmax threshold had the greatest value for tumor recurrence evaluation and exhibited the best consistency with prognosis-related hematological parameters.
As an important imaging method for preoperative evaluation of cervical cancer, 18F-FDG PET/CT imaging can be used to detect the primary tumor and SMLNs. Moreover, the semiquantitative parameters from such imaging (SUVmax, MTV, and TLG), which reflect tumor metabolic activity, are adversely associated with prognosis (7-11). Several researchers (8-11) have reported that higher pretreatment PET parameters for the primary tumor indicate a worse prognosis. However, independent of PET parameters, hypermetabolic LN status on PET has been regarded as a risk factor for tumor recurrence (5-9). New PET parameters that combine the two important prognostic factors from the primary tumor and SMLNs have been proposed and applied, and these parameters have mainly been determined by using two calculation methods (7,12-15). The first calculation method, which involves delineating multiple VOIs of malignant lesions, has been used in certain studies (7,14,15). In this method, the whole-body SUVmax was defined to be the highest SUVmax of all malignant lesions, while the whole-body MTV was defined to be the sum of the MTVs for all malignant lesions. The whole-body TLG was calculated by adding the products of the mean SUV and MTV for all individual lesions. With the second calculation method, which has been investigated by Liang et al. (12) and also in our study (displayed in Figure 1), all malignant lesions were included within one initial region (excluding physiological FDG uptake). SUVmax had the same value as in the first method. MTV and TLG were calculated based on the VOI, which was delineated by using unified SUVmax values from either the primary tumor or higher SMLNs. If a fixed threshold (i.e., SUVmax >2.5) was used to calculate PET parameters (13), there was no difference between the two methods. In agreement with prior studies (12-15), in our investigation, the diagnostic efficiency of PET parameters for all lesions was greater than or equal to that of PET parameters for the primary tumor. MTV-C had greater diagnostic efficiency than SUVmax-C and TLG-C, regardless of which %SUVmax threshold was used. Our findings do not agree with those of Liang et al. (12) in that TLG-C showed the best diagnostic performance (AUC =0.598) in their study (12). This discrepancy may be attributable to several factors. First, larger bias in their investigation was inevitable given their retrospective study design. Second, we included a relatively large number of patients, and 43 of our 89 patients had SMLNs. Third, their nonuniform treatment regimens may have affected patient survival. Finally, different statistical methods were used. In our study, Harrell’s c-index, which considers survival time, was used to evaluate predictive ability. Recently, two others studies (7,14) concluded that MTV-C performed better than TLG-C as a predictor of treatment response and tumor recurrence. Overall, we believe that MTV-C is a better predictor than TLG-C for LACC. Semiquantitative MTV can approximate the number of active metabolic tumor cells, whereas TLG represents a combination of SUVmean and MTV. Compared with early-stage tumors, LACC has greater internal heterogeneity and is more likely to include true tumor tissue, vasculature, inflammatory reaction zones and necrotic zones, causing deviations in SUVmean and therefore reducing the accuracy of TLG.
The most important limitation of previous studies (6,7,11-13,15) involving MTV and TLG calculation was a single-threshold approach, because the choice of threshold influences measurements of MTV, mean SUV, and TLG. A threshold of approximately 40%SUVmax is most commonly used because several studies (23,24) found that the PET-based MTV estimated by using this threshold was similar to the postoperative pathological volume and the calculated volume on T2-weighted MRI. Recently, Scher et al. (16) and Leseur et al. (17) found that the optimal predictive threshold for preoperative primary cervical cancer was 48% and 55%, respectively. The optimal threshold in our study was 50%SUVmax. A 48%SUVmax threshold was not explored due to the use of a larger interval of 5%. The enrollment of patients with early cervical cancer (FIGO stage of less than IIb) by Leseur et al. (17) may have led to differences in results between their study and our own. To the best of our knowledge, our study is unique in proposing a 50%SUVmax threshold as the best threshold for MTV-C and TLG-C for evaluating RFS. A larger cohort in which all patients have SMLNs should be used to validate this finding in the future.
Semiquantitative PET metabolic parameters and hematological parameters are easily accessible, noninvasive indicators for evaluating the prognosis of LACC. PET parameters and hematological parameters may provide complementary information; therefore, combinations of these two types of parameters can be used as joint prognostic factors. A high value of SCC-ag, a widely accepted tumor marker for SCCC, has been shown to indicate poor prognosis (18). Pan et al. (11) concluded that there was no correlation between SUVmax and serum SCC-ag in primary cervical cancer. Another study (25) found that MTV and TLG were significantly higher in subjects with higher serum SCC-ag. We did not discover a correlation between SUVmax and SCC-ag but found that MTV and TLG were moderately correlated with SCC-ag. These results were consistent with previously reported findings. Anemia has long been linked to poor prognosis in patients with cervical cancer (2,19). An alternative explanation for this association is that low Hb levels induce tumor hypoxia (26). Decreased Hb levels reduce oxygen transport capacity and cause decreased tumor cell oxygenation, and both radiotherapy and chemotherapy will be less effective under hypoxic conditions. In this study, correlations between PET parameters and Hb were not found in LACC, possibly due to multiple factors, including nutrition deficiency, tumor bleeding, tumor-induced bone marrow infiltration, and myelosuppressive effects of anticancer therapy, among others. Recently, systemic inflammatory response markers (NLR and PLR), particularly NLR, have also been shown to be associated with tumor prognosis (3,4,20,27). The mechanism underlying the relationship between high NLR (or PLR) and poor prognosis is complicated and remains unclear, but certain opinions may be useful for interpreting the results of our study. Inflammation is known to alter the tumor microenvironment and promote angiogenesis and metastasis. Neutrophils and platelets contain and secrete inflammatory factors that directly contribute to tumor angiogenesis, growth and metastasis, whereas lymphocytes secrete protective inflammatory factors that prevent proliferation and metastasis. In a study of small cell lung cancer (21), NLR was most strongly correlated with primary-tumor MTV, whole-body MTV and TLG. The precise mechanism explaining these correlations is under investigation. One possible explanation may be that inflammatory cells that infiltrate malignant lesions increase FDG uptake. Another potential explanation relates to the induction of angiogenesis by inflammation. The hypoxia of the tumor microenvironment promotes increased inflammatory cells that secrete angiogenic factors, resulting in the production of a large number of new blood vessels and increased FDG uptake in the tumor. The relationship between local FDG metabolism and systemic inflammatory response merits further investigation because of the coexistence of and interaction between true tumor tissue and the inflammatory zone. In our study, compared with PET parameters for the primary tumor alone, PET parameters based on the whole-body tumor burden were more strongly related to NLR and PLR. To the best of our knowledge, our investigation is the first study to evaluate how NLR and PLR are correlated with PET parameters with different thresholds for LACC. No significant correlation was found between SUVmax and NLR or PLR, but with most thresholds, MTV and TLG exhibited slightly to moderately significant correlations with NLR and PLR. Similar to our findings for evaluating tumor recurrence, we determined that MTV-C50%, a preferable parameter for reflecting metabolic activity of the tumor core, also had the highest correlation coefficients with NLR and PLR (r=0.637 and r=0.515, respectively; P<0.001 for both correlations; Table 3).
Our preliminary results proposed assessing LACC patients comprehensively based on whole-body PET parameters estimated with a 50%SUVmax threshold and systemic inflammatory response biomarkers. Prior to treatment, relatively high values of MTV-C50% (≥23.04 mL) and NLR (≥3.28) and the presence of SMLNs indicated short RFS. However, these cutoff values need to be further verified using a large cohort. Our study has some limitations. First, without pathological confirmation, all hypermetabolic LNs were automatically delineated and included in the VOI. Certain inflammatory LNs showed reactive hypermetabolism, and it could not be determined whether this phenomenon was indicative of inflammation or metastasis. Second, the first calculation method described above, which involves the mathematical summation of all individual lesions, was not applied or compared with our approach in this study. Additionally, our study only covered traditional PET parameters, although some researchers (28,29) had mined radiomics features of PET, which is what we will focus on in future studies. Finally, only an adaptive threshold method was employed in our study with the purpose of exploring the optimal %SUVmax threshold; the fixed threshold method should be utilized for the validation analysis in future studies.
Conclusions
The MTV-C estimated by using a 50%SUVmax threshold may be an optimal PET parameter associated with tumor recurrence for LACC and may help identify patients at high risk of recurrence. The slight-to-moderate correlations between PET parameters (MTV and TLG) and SCC-ag, NLR, and PLR suggest that pretreatment hematological parameters and PET metabolic parameters offer complementary information for evaluating tumor recurrence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Shengjing Hospital of China Medical University Technology Ethics Committee (No. 2014PS144J) and written informed consent was obtained from all patients.
References
- Spensley S, Hunter RD, Livsey JE, Swindell R, Davidson SE. Clinical outcome for chemoradiotherapy in carcinoma of the cervix. Clin Oncol (R Coll Radiol) 2009;21:49-55. [Crossref] [PubMed]
- Kudaka W, Nagai Y, Toita T, Inamine M, Asato K, Nakamoto T, Wakayama A, Ooyama T, Tokura A, Murayama S, Aoki Y. Long-term results and prognostic factors in patients with stage III–IVA squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy from a single institution study. Int J Clin Oncol 2013;18:916-21. [Crossref] [PubMed]
- Zhu M, Feng M, He F, Han B, Ma K, Zeng X, Liu Z, Liu X, Li J, Cao H, Liang Y, Jia C, Zhang L. Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer. Clin Chim Acta 2018;483:296-302. [Crossref] [PubMed]
- Onal C, Guler OC, Yildirim BA. Prognostic use of pretreatment hematologic parameters in patients receiving definitive chemoradiotherapy for cervical cancer. Int J Gynecol Cancer 2016;26:1169-75. [Crossref] [PubMed]
- Guler OC, Torun N, Yildirim BA, Onal C. Pretreatment metabolic tumor volume and total lesion glycolysis are not independent prognosticators for locally advanced cervical cancer patients treated with chemoradiotherapy. Br J Radiol 2018;91:20170552. [Crossref] [PubMed]
- Akkas BE, Demirel BB, Dizman A, Vural GU. Do clinical characteristics and metabolic markers detected on positron emission tomography/computerized tomography associate with persistent disease in patients with in-operable cervical cancer? Ann Nucl Med 2013;27:756-63. [Crossref] [PubMed]
- Lima GM, Matti A, Vara G, Dondi G, Naselli N, De Crescenzo EM, Morganti AG, Perrone AM, De Iaco P, Nanni C, Fanti S. Prognostic value of posttreatment 18F-FDG PET/CT and predictors of metabolic response to therapy in patients with locally advanced cervical cancer treated with concomitant chemoradiation therapy: an analysis of intensity- and volume-based PET parameters. Eur J Nucl Med Mol Imaging 2018;45:2139-46. [Crossref] [PubMed]
- Yoo J, Choi JY, Moon SH, Bae DS, Park SB, Choe YS, Lee KH, Kim BT. Prognostic significance of volume-based metabolic parameters in uterine cervical cancer determined using 18F-fluorodeoxyglucose positron emission tomography. Int J Gynecol Cancer 2012;22:1226-33. [Crossref] [PubMed]
- Cima S, Perrone AM, Castellucci P, Macchia G, Buwenge M, Cammelli S, Cilla S, Ferioli M, Ferrandina G, Galuppi A, Salizzoni E, Rubino D, Fanti S, De Iaco P, Morganti AG. Prognostic impact of pretreatment fluorodeoxyglucose positron emission tomography/computed tomography SUVmax in patients with locally advanced cervical cancer. Int J Gynecol Cancer 2018;28:575-80. [Crossref] [PubMed]
- Krhili S, Muratet JP, Roche S, Pointreau Y, Yossi S, Septans AL, Denis F. Use of metabolic parameters as prognostic factors during concomitant chemoradiotherapy for locally advanced cervical cancer. Am J Clin Oncol 2017;40:250-5. [Crossref] [PubMed]
- Pan L, Cheng J, Zhou M, Yao Z, Zhang Y. The SUVmax (maximum standardized uptake value for F-18 fluorodeoxyglucose) and serum squamous cell carcinoma antigen (SCC-ag) function as prognostic biomarkers in patients with primary cervical cancer. J Cancer Res Clin Oncol 2012;138:239-46. [Crossref] [PubMed]
- Liang Y, Li X, Wan H, Fang Y, Zheng R, Zhang W, Liu Y, Chen C, Wu N. Prognostic value of volume-based metabolic parameters obtained by 18F-FDG–PET/CT in patients with locally advanced squamous cell cervical carcinoma. J Comput Assist Tomogr 2018;42:429-34. [Crossref] [PubMed]
- Hong JH, Min KJ, Lee JK, So KA, Jung US, Kim S, Eo JS. Prognostic value of the sum of metabolic tumor volume of primary tumor and lymph nodes using 18F-FDG PET/CT in patients with cervical cancer. Medicine (Baltimore) 2016;95:e2992. [Crossref] [PubMed]
- Chong GO, Lee WK, Jeong SY, Park SH, Lee YH, Lee SW, Hong DG, Kim JC, Lee YS. Prognostic value of intratumoral metabolic heterogeneity on F-18 fluorodeoxyglucose positron emission tomography/computed tomography in locally advanced cervical cancer patients treated with concurrent chemoradiotherapy. Oncotarget 2017;8:90402-12. [Crossref] [PubMed]
- Sun Y, Lu P, Yu L. The volume-metabolic combined parameters from 18F-FDG PET/CT may help predict the outcomes of cervical carcinoma. Acad Radiol 2016;23:605-10. [Crossref] [PubMed]
- Scher N, Castelli J, Depeursinge A, Bourhis J, Prior JO, Herrera FG, Ozsahin M. (18F)-FDG PET/CT parameters to predict survival and recurrence in patients with locally advanced cervical cancer treated with chemoradiotherapy. Cancer Radiother 2018;22:229-35. [Crossref] [PubMed]
- Leseur J, Roman-Jimenez G, Devillers A, Ospina-Arango JD, Williaume D, Castelli J, Terve P, Lavoue V, Garin E, Lejeune F, Acosta O, De Crevoisier R. Pre- and per-treatment 18F-FDG PET/CT parameters to predict recurrence and survival in cervical cancer. Radiother Oncol 2016;120:512-8. [Crossref] [PubMed]
- Hirakawa M, Nagai Y, Inamine M, Kamiyama K, Ogawa K, Toita T, Murayama S, Aoki Y. Predictive factor of distant recurrence in locally advanced squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy. Gynecol Oncol 2008;108:126-9. [Crossref] [PubMed]
- Koulis TA, Kornaga EN, Banerjee R, Phan T, Ghatage P, Magliocco AM, Lees-Miller SP, Doll CM. Anemia, leukocytosis and thrombocytosis as prognostic factors in patients with cervical cancer treated with radical chemoradiotherapy: a retrospective cohort study. Clin Transl Radiat Oncol 2017;4:51-56. [Crossref] [PubMed]
- Wang YY, Bai ZL, He JL, Yang Y, Zhao R, Hai P, Zhe H. Prognostic value of neutrophil-related factors in locally advanced cervical squamous cell carcinoma patients treated with cisplatin-based concurrent chemoradiotherapy. Dis Markers 2016;2016:3740794. [Crossref] [PubMed]
- Mirili C, Guney IB, Paydas S, Seydaoglu G, Kapukaya TK, Ogul A, Gokcay S, Buyuksimsek M, Yetisir AE, Karaalioglu B, Tohumcuoglu M. Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET–CT metabolic parameters in small cell lung cancer (SCLC). Int J Clin Oncol. 2019;24:168-78. [Crossref] [PubMed]
- Sürücü E, Demir Y, Şengöz T. The correlation between the metabolic tumor volume and hematological parameters in patients with esophageal cancer. Ann Nucl Med 2015;29:906-10. [Crossref] [PubMed]
- Zhang Y, Hu J, Li J, Wang N, Li W, Zhou Y, Liu J, Wei L, Shi M, Wang S, Wang J, Li X, Ma W. Comparison of imaging-based gross tumor volume and pathological volume determined by whole-mount serial sections in primary cervical cancer. Onco Targets Ther 2013;6:917-23. [PubMed]
- Sun H, Xin J, Zhang S, Guo Q, Lu Y, Zhai W, Zhao L, Peng W, Wang B. Anatomical and functional volume concordance between FDG PET, and T2 and diffusion weighted MRI for cervical cancer: a hybrid PET/MR study. Eur J Nucl Med Mol Imaging 2014;41:898-905. [Crossref] [PubMed]
- Xu W, Yu S, Xin J, Guo Q. Relationship of 18F-FDG PET/CT metabolic, clinical and pathological characteristics of primary squamous cell carcinoma of the cervix. J Investig Med 2016;64:1246-51. [Crossref] [PubMed]
- Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist 2004;9:31-40. [Crossref] [PubMed]
- Temur I, Kucukgoz Gulec U, Paydas S, Guzel AB, Sucu M, Vardar MA. Prognostic value of pre-operative neutrophil/lymphocyte ratio, monocyte count, mean platelet volume, and platelet/lymphocyte ratio in endometrial cancer. Eur J Obstet Gynecol Reprod Biol 2018;226:25-9. [Crossref] [PubMed]
- Fujima N, Hirata K, Shiga T, Yasuda K, Onimaru R, Tsuchiya K, Kano S, Mizumachi T, Homma A, Kudo K, Shirato H. Semi-quantitative analysis of pre-treatment morphological and intratumoral characteristics using 18F-fluorodeoxyglucose positron-emission tomography as predictors of treatment outcome in nasal and paranasal squamous cell carcinoma. Quant Imaging Med Surg 2018;8:788-95. [Crossref] [PubMed]
- Lucia F, Visvikis D, Desseroit MC, Miranda O, Malhaire JP, Robin P, Pradier O, Hatt M, Schick U. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging 2018;45:768-86. [Crossref] [PubMed]


