
Left ventricular longitudinal strain is associated with mitral annular fractional area change in healthy subjects—Results from the three-dimensional speckle tracking echocardiographic MAGYAR-Healthy Study
Introduction
The mitral valve (MV) apparatus is a complex three-dimensional (3D) functional unit which separates the left atrium and the left ventricle (LV), and has a critical role in the regulation of normally unidirectional blood flow (1). Compared to the LV ejection fraction (EF), LV longitudinal systolic function was found to be more sensitive in the detection of cardiac depression in several disorders (2,3). Three-dimensional speckle-tracking echocardiography (3DSTE) is a clinical tool of choice for simultaneous quantification of longitudinal LV deformation and mitral valve (MV) morphology and function (4,5). LV-MV interactions are not clearly understood at this moment, therefore the relationship between LV quantitative features of longitudinal contractility and MA size and function was aimed to be investigated in healthy subjects in this study.
Methods
Subjects
The present study comprised 295 healthy adults; 117 subjects were excluded due to inferior image quality (40%). Finally, 178 healthy adults (mean age: 32.0±11.3 years, 92 males) have been included without risk factors, known diseases or other conditions which theoretically could have affected the results. None of them take any medications at the time of examination. Complete two dimensional (2D) Doppler echocardiography and 3DSTE were performed in all cases at the same time by the same echocardiography machines. The results of the present study are a part of the MAGYAR-Healthy Study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Healthy subjects). It was organized at the Cardiology Center of the University of Szeged to assess the clinical role of 3DSTE-derived echocardiographic parameters in healthy subjects (‘magyar’ means ’Hungarian’ in Hungarian language) among others. Informed consent was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Two-dimensional echocardiography
Toshiba ArtidaTM (Toshiba Medical Systems, Tokyo, Japan) echocardiography equipment using a PST-30SBP (1–5 MHz) phased-array transducer was used for standard transthoracic Doppler examinations. Concerning guidelines, LV-EF was calculated by the Simpson’s method (6). Visual grading and Doppler assessments were used to exclude valvular abnormalities.
3DSTE-derived data acquisition and quantitative analysis
The same Toshiba ArtidaTM echocardiography equipment was used with a fully sampled PST-25SX matrix-array transducer (Toshiba Medical Systems, Tokyo, Japan) with 3D capability. Pyramidal 3D full volumes were formed by the software using the R-wave triggered LV subvolumes from the 3D pyramidal data acquired during six consecutive cardiac cycles during one breath-hold from apical views in accordance with recent practices (4,7). Depth and angle were adjusted for optimal temporal and spatial resolution. 3D Wall Motion Tracking software version 2.7 (Toshiba ArtidaTM; Toshiba Medical Systems, Tokyo, Japan) was used for quantitative analysis.
3DSTE-derived LV longitudinal strain assessments
Three short-axis views acquired at different LV levels and the apical two- (AP2CH) and four-chamber (AP4CH) views were selected automatically from the 3D echocardiographic pyramidal dataset at end-diastole by the software (4,7). For 3D reconstruction of the endocardial surface, the examiner selected two points at the edges of the mitral valve and one point at the apex on the 3D reconstruction of the endocardial surface on the AP2CH and AP4CH views. Then the endocardial surface was manually adjusted in all apical long- and short-axis. After these adjustments were made, the software automatically reconstructed and tracked the endocardial surface in 3D space throughout the cardiac cycle, and generated curves for global and mean segmental LV longitudinal strains (LSs) (Figure 1). LS is expressed as a negative number.
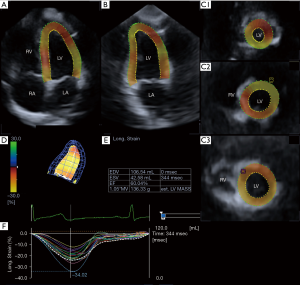
3DSTE-derived mitral annular measurements
MA measurements were performed in accordance with a simple method recently demonstrated by our working group. Briefly, from short-axis views, C3 was positioned at the level of the MA with the help of AP2CH and AP4CH views to find the optimal endpoints of the MA at end-diastole and end-systole (Figure 2) (5):
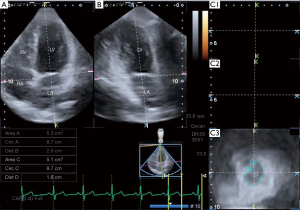
MA morphologic parameters
- MA diameter (MAD) was defined as the perpendicular line drawn from the peak of MA curvature to the middle of the straight MA border both at end-diastole, just before mitral valve closure, and at end-systole, just before mitral valve opening;
- MA area (MAA) was measured by planimetry both at end-diastole and at end-systole;
- MA perimeter (MAP) was measured by planimetry both at end-diastole and at end-systole.
MA functional parameters
- MA fractional shortening (MAFS) = (end-diastolic MAD − end-systolic MAD) / (end-diastolic MAD × 100);
- MA fractional area change (MAFAC) = (end-diastolic MAA − end-systolic MAA) / (end-diastolic MAA × 100).
Statistical analysis
All values were expressed as mean ± standard deviation. Group comparisons were made with unpaired Student’s t-test. For the dichotomous variables, chi-square analysis and Fisher’s exact test were performed. Two-sided P<0.05 was defined as statistical significance. Pearson’s coefficient was calculated to examine correlations between parameters featuring MA morphology and global and mean segmental LV-LS and for intraobserver and interobserver correlations. Inter- and intraobserver reproducibility of measurements of end-diastolic and end-systolic MA parameters and LV-LS was tested in healthy subjects. To assess the predictive power of MAFAC, receiver operator curve (ROC) was constructed and the area under the curve was reported with sensitivity and specificity values with 95% confidence intervals. MedCalc software was used for statistical calculations (MedCalc, Mariakerke, Belgium).
Results
Clinical data
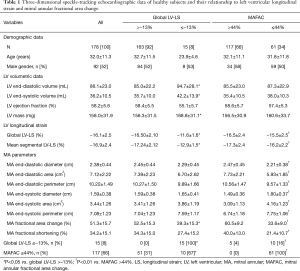
Demographic data are presented in Table 1. Average height and weight proved to be 172.3±11.1 cm and 71.8±17.7 kg, respectively.
Full table
Two-dimensional echocardiographic data
Routine two-dimensional Doppler echocardiography showed normal results including left atrial diameter (36.8±4.0 mm), interventricular septum thickness (9.1±1.5 mm), systolic and diastolic LV diameter (38.4±23.5 and 48.0±3.9 mm, respectively) and LV volume (36.4±9.1 and 97.4±29.4 mL, respectively), LV ejection fraction (65.6%±4.7%), transmitral flow velocity E (72.8±26.0 cm/s) and A (64.3±19.0 cm/s) and their ratio (1.25±0.52). None of the healthy subjects had grade ≥1 valvular regurgitation or significant valvular stenosis.
Three-dimensional speckle-tracking echocardiographic data
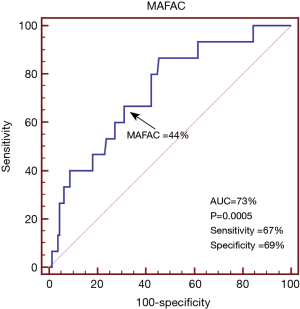
The global and mean segmental LV-LS proved to be −16.1%±2.5% and −16.9%±2.4%, respectively (Table 1). 3DSTE-derived LV volumetric, LV-LS and MA data are presented in Table 1. In the present study, LV-LS ≤−13% was considered to be reduced. In ROC analysis, the cut-off value for MAFAC to predict impaired LV-LS was ≤44%, with 67% sensitivity (95% CI, 38–88%) and 69% specificity (95% CI, 61–76%) and ROC area under curve 0.73 (P=0.0005) (Figure 3). Significantly increased LV volumes and LV mass and reduced MAFAC could be demonstrated in healthy subjects with ≤−13% global LV-LS as compared to cases with global LV-LS >−13%. Significantly larger ratio of subjects with global LV-LS ≤−13% had MAFAC ≤44% as compared to cases with >−13% global LV-LS (31% vs. 67%, P=0.009). Patients with MAFAC ≤44% had significantly reduced global and mean segmental LV-LS, reduced end-diastolic and increased end-systolic MA sizes and reduced MAFS. Significantly larger ratio of subjects with MAFAC ≤44% had global LV-LS ≤−13% as compared to cases with MAFAC >44% (4% vs. 16%, P=0.009). MAFAC showed no correlations with global or mean segmental LV-LS.
Reproducibility measurements
Table 2 shows the mean ± standard deviation difference in values obtained by two measurements of the same observer and two observers for the measurements of 3DSTE-derived end-diastolic and end-systolic MA parameters and LV-LS in 20 healthy subjects, along with the respective correlation coefficients.

Full table
Discussion
The normal MV apparatus is a dynamic 3D structure which allows normal blood flow during the cardiac cycle: LV inflow from the LA during diastole and LV outflow into the aorta during systole. The key components are the MA, the valve leaflets, the chordae tendineae, and the LV wall with its attached papillary muscles (1). MA plays a significant role in promoting LA and LV filling and emptying, which is dependent on LV functional properties (8). LV strains are quantitative features of LV myocardial contractility. Global LV-LS characterizes LV deformation (lengthening or shortening) in longitudinal direction and has good prognostic value in various disorders (9,10). Moreover, LV-LS is more sensitive than LV-EF in detecting abnormalities in LV systolic function as noted by Carasso et al. (3). 3DSTE allows complete non-invasive assessment of the heart chambers in 3D space including parallel evaluation of MA and LV morphology and function (at the same time) from the same 3D dataset. This advantage enables physiologic studies assessing the effects of these components on each other.
In recent studies global LV-LS and MV function have been demonstrated to be associated in several pathological states (11). However, to the best of the authors’ knowledge, no clinical studies are available directly assessing a relationship between LV longitudinal deformation and MA functional properties in healthy subjects. Lower global LV-LS was found to be associated with lower MA function. Moreover, impaired LV longitudinal deformation proved to have a prognostic role in the prediction of MAFAC, as well. These results could suggest that subclinical impairment of LV longitudinal function is associated with reduced MA function in otherwise healthy subjects without risk factors or overt cardiovascular diseases. This result is against what could be demonstrated in different disorders. For instance, although type 1 diabetes mellitus is associated with impaired global LV-LS (12), MA functional properties proved to be significantly increased suggesting a compensatory mechanism in these patients (5). Our results suggest that demand for this compensatory mechanism did not reach a certain level required for the mechanism to develop in our healthy subject. Further studies are warranted to confirm our findings and to reveal pathophysiological background of this compensation.
Limitation section
The most important limitations occurring during the 3DSTE studies are listed below:
- Spatial and temporal resolution of the relatively new 3DSTE is low which could affect the results and should be considered when interpreting the results.
- Although 3DSTE could measure LV volumes, its accuracy depends on the quality of the acquired image and particularly on enlargement of the LV (13). This study tried to mirror real life experience, therefore 3DSTE-derived LV volumetric data could be somewhat lower as expected.
- Early stage diseases were not excluded by other imaging or laboratory tests, although lower strain values could indicate subclinical changes.
- LV strain and volumetric and functional data of heart chambers other than the LV were not examined in this study.
- Although the MA geometric shape approximates a hyperbolic paraboloid, only one plane MA motion and function was analysed in this study.
- Spatial longitudinal analysis of the MA movement along its long-axis is also possible (6), it was not aimed to be measured and compared to other parameters in this particular study.
Conclusions
There is a strong relationship between MA and LV longitudinal function. MA fractional area change predicts global LV-LS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of the University of Szeged. Informed consent was obtained from each patient.
References
- Dal-Bianco JP, Levine RA. Anatomy of the Mitral Valve Apparatus – Role of 2D and 3D Echocardiography. Cardiol Clin 2013;31:151-64. [Crossref] [PubMed]
- Zhang HM, Wang XT, Zhang LN, He W, Zhang Q, Liu DW. Chinese Critical Ultrasound Study Group. Left Ventricular Longitudinal Systolic Function in Septic Shock Patients with Normal Ejection Fraction: A Case-control Study. Chin Med J (Engl) 2017;130:1169-74. [Crossref] [PubMed]
- Carasso S, Cohen O, Mutlak D, Adler Z, Lessick J, Reisner SA, Rakowski H, Bolotin G, Agmon Y. Differential effects of afterload on left ventricular long- and short-axis function: Insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am Heart J 2009;158:540-5. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography -- a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [Crossref] [PubMed]
- Nemes A, Piros GÁ, Domsik P, Kalapos A, Lengyel C, Várkonyi TT, Orosz A, Forster T. Changes in mitral annular morphology and function in young patients with type 1 diabetes mellitus-results from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2015;5:815-21. [PubMed]
- Nemes A, Forster T. Recent echocardiographic examination of the left ventricle – from M-mode to 3D speckle-tracking imaging. Orv Hetil 2015;156:1723-40. [Crossref] [PubMed]
- Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, Gorissen W, Houle H. Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography 2013;30:88-105. [Crossref] [PubMed]
- Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J 2012;164:163-76. [Crossref] [PubMed]
- Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and Strain Rate Imaging by Echocardiography – Basic Concepts and Clinical Applicability. Curr Cardiol Rev 2009;5:133-48. [Crossref] [PubMed]
- Ng ACT, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Ajmone Marsan N, Leung DY, Delgado V, Bax JJ. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging 2018;19:859-67. [Crossref] [PubMed]
- Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, Versteegh MI, Holman ER, Schalij MJ, Bax JJ, Klautz RJ, Marsan NA. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging 2013;14:69-76. [Crossref] [PubMed]
- Jensen MT, Risum N, Rossing P, Jensen JS. Self-reported dyspnea is associated with impaired global longitudinal strain in ambulatory type 1 diabetes patients with normal ejection fraction and without known heart disease - The Thousand & 1 Study. J Diabetes Complications 2016;30:928-34. [Crossref] [PubMed]
- Kawamura R, Seo Y, Ishizu T, Atsumi A, Yamamoto M, Machino-Ohtsuka T, Nakajima H, Sakai S, Tanaka YO, Minami M, Aonuma K. Feasibility of left ventricular volume measurements by three-dimensional speckle tracking echocardiography depends on image quality and degree of left ventricular enlargement: validation study with cardiac magnetic resonance imaging. J Cardiol 2014;63:230-8. [Crossref] [PubMed]


