
Increased serum malondialdehyde levels are associated with grey matter volume loss in patients with non-alcoholic cirrhosis
Introduction
Liver cirrhosis is commonly accompanied by not only cerebral functional disturbances but also cerebral morphological abnormalities (1-3). Accumulating evidence has shown the presence of brain grey matter volume (GMV) loss and neuronal cell death, which are regarded as the basis of an irreversible neurocognitive impairment, in cirrhotic patients (4-7). The mechanisms underlying GMV loss in cirrhosis remain unclear. However, a more noticeable GMV loss has been observed in patients with alcoholic cirrhosis, a history of overt hepatic encephalopathy (HE) and more severe liver dysfunction (2,5). Surprisingly, ammonia, which is generally thought to be the primary cause of cirrhosis-related neurocognitive impairment, was the least powerful predictor of abnormalities in cerebral morphology in a computed tomography (CT) study (3). Moreover, ammonia was found to have no correlation with GMV loss in magnetic resonance imaging (MRI) studies (4,5).
Oxidative stress (OS) is defined as an imbalance between oxidants and antioxidants accompanied by overproduction of reactive oxygen species (ROS) (8). OS results in the production of oxidation products and the depletion of endogenous antioxidants. Excessive ROS damage cellular structures and macromolecules, leading to cellular dysfunction and ultimately cell death (9). The brain is particularly vulnerable to oxidative damage due to its high metabolic rate, limited antioxidant capacity, and rich content of easily oxidizable polyunsaturated fatty acids (10). OS is known to contribute to brain aging and neurodegenerative diseases (11,12) which are characterized by brain atrophy and GMV loss. Moreover, studies have reported a relationship between oxidative damage and GMV loss in a variety of clinical populations, such as patients with chronic human immunodeficiency virus infection, diabetes, and psychosis (13-15).
OS is a recognized feature of chronic liver disease (CLD), which is not restricted to the ailing liver but represents a systemic phenomenon (16,17). Malondialdehyde (MDA) is a product of lipid peroxidation and a sensitive and reliable biomarker of oxidative tissue damage (18). High levels of MDA have been detected in blood samples from cirrhotic patients in many studies (19-21). Furthermore, postmortem studies have revealed a great deal of lipofuscin pigment (indicating peroxidized lipids) in the brains of cirrhotic patients (22).
Based on the previously mentioned studies investigating GMV loss and OS in cirrhosis, we hypothesized that GMV loss observed in cirrhosis might be related to oxidative damage. Therefore, we evaluated the relationship between an oxidative stress marker (MDA) and GMV loss in patients with non-alcoholic cirrhosis.
Methods
Participants
Between August 2016 and July 2017, patients with non-alcoholic cirrhosis attending the Hepatology Outpatient Department of the Third Xiangya Hospital were enrolled in this prospective study. Thirty-four patients with non-alcoholic cirrhosis (mean age 49.29±9.48 years; 28 males, 6 females) were included in this study. The diagnosis of cirrhosis was based on medical history and physical, biochemical and imaging examinations. Since previous studies have shown that alcohol has a direct effect on the brain (23) and since it is difficult to distinguish the neurological consequences of alcohol and CLD, we excluded patients with alcoholic cirrhosis. Due to poor compliance, patients with overt HE or a history of overt HE were also excluded. Other exclusion criteria for cirrhotic patients included recent (<1 month) infection or gastrointestinal bleeding, a surgical portocaval shunt or a transjugular intrahepatic portosystemic shunt, or hepatic malignancy.
Twenty-seven age- and sex-matched healthy controls (mean age 46.85±7.85 years; 20 males, 7 females) without liver diseases were recruited from the local community.
Exclusion criteria for all subjects included alcoholism, history of drug abuse, treatment with drugs that would induce an antioxidant/pro-oxidant status imbalance, history of severe head trauma, neurological or psychiatric disorder, hypertension, diabetes, kidney failure, cerebrovascular disease or any lesion detected on conventional brain MRI.
Clinical methods
All subjects underwent physical examinations and blood biochemistry tests on the same day as the MRI scan.
Blood draws were performed on subjects at 7:00 am after a night of fasting with water allowed. Blood biochemistry tests included liver function tests, kidney function tests, coagulation tests, and plasma ammonia tests. The degree of liver failure in the patient group was determined according to the Child-Pugh classification.
Measurement of serum MDA levels
Blood samples used for the determination of MDA were fasting morning samples. Venous blood (5 mL) was taken and centrifuged. The supernatant was collected and stored at −80 °C. All samples were analyzed on the same day. Serum MDA levels were measured using a Lipid Peroxidation (MDA) Assay Kit (Sigma).
Brain MRI
All subjects were scanned with a 3T scanner (Ingenia, Philips) using a phased array coil. An ultrafast gradient echo 3D sequence (T1W_3D_TFE_ref) was used. Scans were acquired in the sagittal orientation with the following parameters: repetition time =7.8 ms, echo time =3.5 ms, inversion time =969.3 ms, gap =0 mm, flip angle =8°, bandwidth = 191.5 Hz/pixel, field of view =240×240 mm2, matrix =240×240, 182 slices with resolution =1×1×1 mm3, and acquisition time = 6 min 26 s. T1-weighted and T2-weighted fast-spin echo images were also obtained to exclude any pathological findings. Quality assurance was performed by an experienced radiologist.
Image analysis
Images were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) in a MATLAB environment (MathWorks Inc., Natick, MA). We used the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) for image segmentation and spatial normalization. After segmentation, we obtained global tissue volumes, including global GMV, global white matter volume and global cerebrospinal fluid volume, in native space. The sum of global GMV, global white matter volume and global cerebrospinal fluid volume was defined as total intracranial volume. We calculated the normalized global GMV by dividing the global GMV of each subject by his/her respective total intracranial volume to normalize the head size of each subject.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). All results are expressed as mean ± standard deviation. Independent sample t-tests were used to detect the significance of age, serum MDA levels and data of kidney function tests between cirrhotic patients and healthy controls; Mann-Whitney U tests were applied to identify the significance for plasma ammonia levels and data of coagulation and liver function tests between the two groups; while Chi-square test was performed to identify the gender significance between the two groups.
Global GMV is related to age and sex (24). Therefore, analysis of covariance with age and sex as covariates was performed to assess the difference in normalized global GMV between the two groups.
Previous studies have revealed that GMV correlates with the degree of liver failure in cirrhotic patients (2,5). Thus, to avoid any confounding effect, we performed a partial correlation analysis in the patient group to investigate the relationship between serum MDA levels and normalized global GMV adjusted for age, sex and Child-Pugh class. We also examined the relationship between plasma ammonia levels and normalized global GMV using a partial correlation analysis.
In addition, a Spearman correlation analysis was performed to examine the relationship between serum MDA and plasma ammonia in the patient group.
A P value of less than 0.05 (uncorrected) was considered to indicate a statistically significant difference.
Results
Demographic and clinical characteristics
Demographic and clinical characteristics are listed in Table 1. There was no significant difference in age or sex between the two groups. Liver function was graded as Child-Pugh A in 19 patients, B in 11 patients and C in 4 patients. None of the patients showed clinical manifestations of overt HE at the time of investigation or had experienced previous episodes of overt HE. Compared with control subjects, cirrhotic patients showed higher plasma ammonia levels (35.53±10.09 µmol/L for patients vs. 14.78±4.45 µmol/L for controls; P<0.001) (Figure 1A).
Full table

Serum MDA levels
Compared with control subjects, cirrhotic patients showed higher serum MDA levels (0.36±0.04 nmol/mL for patients vs. 0.17±0.04 nmol/mL for controls; P<0.001) (Figure 1B).
Global GMV
Analysis of covariance revealed a significant difference in normalized global GMV between the two groups, with patients exhibiting a smaller normalized global GMV than healthy controls (0.43±0.02 for patients vs. 0.44±0.01 for controls; P=0.042) (Figure 1C).
Correlations between normalized global GMV, serum MDA levels and plasma ammonia levels in the patient group
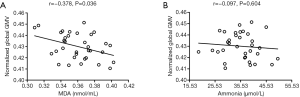
In the patient group, partial correlation analysis revealed a significant negative correlation between normalized global GMV and serum MDA levels (r=−0.378, P=0.036, uncorrected) after adjusting for age, sex and Child-Pugh class (Figure 2A). There was no significant correlation between normalized global GMV and plasma ammonia levels (r=−0.097, P=0.604, uncorrected) (Figure 2B).
No significant correlation was found between serum MDA levels and plasma ammonia levels in the patient group (r=0.241, P=0.169, uncorrected).
Discussion
In this study, we evaluated the relationship between serum MDA levels and global GMV in patients with non-alcoholic cirrhosis. Our main finding was that increased serum MDA levels were associated with GMV loss in patients with non-alcoholic cirrhosis. To the best of our knowledge, this study is the first to investigate the relationship between oxidative damage and GMV loss in cirrhosis. Although the association we detected does not imply causation, we provide a reasonable hypothesis that OS adversely affects GMV in cirrhotic patients.
Neuroimaging plays an important role in uncovering cerebral morphological and functional abnormalities (25,26). Brain atrophy and GMV loss in cirrhotic patients are robust findings assessed by CT and MRI (2-6). As expected, cirrhotic patients exhibited a smaller normalized global GMV than healthy controls in the present study, which is in line with the results of previous studies. However, GMV loss observed in cirrhotic patients seems contradictory to previous MR spectroscopy (MRS) studies. Most MRS studies have found no significant difference in N-acetylaspartate, which is a neuronal marker, between cirrhotic patients and healthy controls (27,28). Zhang et al. speculated that this discrepancy might be due to the region of interest selected in MRS studies (the spectrum was obtained from one or two specific areas of 8 mm3 in volume, such as white matter regions and the anterior cingulate cortex), which may lead to false-negative results (5).
Evaluating MDA is a traditional method to detect oxidative damage in various tissues. In the present study, a significant increase in serum MDA levels in cirrhotic patients was reliable evidence of OS in these patients. Numerous studies have confirmed the presence of OS in cirrhotic patients using various peripheral blood markers (16,19-21). Therefore, the results of our study are consistent with previous observations that OS is a remarkable feature of cirrhosis.
In this study, no significant correlation was found between serum MDA and plasma ammonia in the patient group. This finding suggests that chronic mild/moderate hyperammonemia does not lead to OS. Observations in portacaval shunted rats support our conclusions as follows: portacaval shunted rats developing hyperammonemia in the absence of intrinsic hepatocellular disease exhibited no signs of OS in either the systemic circulation or the central nervous system (29).
The present study revealed a significant negative correlation between serum MDA levels and normalized global GMV in cirrhotic patients after adjusting for age, sex and Child-Pugh class. This correlation supports our hypothesis that OS plays a role in GMV loss in cirrhosis. The brain is prone to OS and does not have sufficient antioxidant capacity to prevent ‘ongoing’ oxidative damage (10). Excessive ROS produced by OS are highly toxic to neurons and interact with biomolecules, leading to lipid peroxidation, DNA and RNA mutations, and protein aggregation. These changes result in neuronal dysfunction and, inevitably, neuron death (30). Moreover, lipid peroxidation products are toxic and have been shown to play a role in neuronal degeneration (31).
Furthermore, data from cirrhotic patients support our finding of a link between OS and GMV loss in cirrhosis. A great deal of lipofuscin pigment (indicating peroxidized lipids) has been observed in the brains of cirrhotic patients (22), providing direct evidence of the involvement of OS in cirrhosis-related brain impairment. Moreover, Montoliu et al. found that serum 3-nitro-tyrosine, a marker of OS, was a good peripheral biomarker of minimal HE in cirrhotic patients (32). Gimenez-Garzó et al. showed increased OS in the blood of cirrhotic patients and significant correlations between the levels of OS markers in blood and deficits in cognitive function and motor coordination (33). These correlations between disturbances in OS indices and brain dysfunction also suggest that OS plays a role in cirrhosis-related brain impairment. However, the studies mentioned above did not explore the correlation between OS indices and abnormalities in cerebral morphology in cirrhosis.
One potential question is whether OS markers in peripheral blood reflect OS in the brain. As shown in the study by Ljubuncic et al. of rats with chronic bile duct ligation, MDA levels increased not only in the liver and blood but also in the brain (17). The authors confirmed that OS in CLD is a systemic phenomenon encompassing tissues and organs throughout the body, even the brain separated by the blood-brain barrier. Therefore, our results of serum MDA levels in cirrhotic patients may at least partially reflect the oxidative status in their brains. Taken together, the observed negative correlation between serum MDA levels and normalized global GMV in cirrhotic patients seems logical.
Similar to previous studies (3-5), we did not found a significant correlation between plasma ammonia levels and normalized global GMV in cirrhotic patients. This finding may initially appear surprising because ammonia is generally believed to play a central role in cirrhosis-related brain impairment. However, it should be noted that a direct correlation between plasma ammonia levels and the severity of neurocognitive impairment is not found in CLD (34). Moreover, in cultured neurons, ammonia-induced neuron apoptosis has been demonstrated to be dose dependent and to reach statistical significance at only 2 mmol/L ammonia or higher (35). In the present study, the mean blood ammonia level in patients was 35.53±10.09 µmol/L, which is far below 2 mmol/L. Similarly, in the studies by Tarter and Zhang, the mean blood ammonia level in patients did not exceed twice the normal level, and no significant correlation was found between brain atrophy or GMV loss and blood ammonia levels (3-5). Thus, we speculate that in cirrhosis, mild/moderate elevations in blood ammonia may not be the primary cause of GMV loss.
This study had several limitations. First, the study employed a small sample size and a cross-sectional design. Therefore, although we identified a relationship between increased serum MDA levels and GMV loss in cirrhotic patients, we cannot conclude that a causal relationship exists. For the same reason, we cannot rule out the possibility that mild hyperammonemia may have had a cumulative effect on GMV loss. A study using a longitudinal design could be more appropriate for determining these relationships. Second, the pathophysiology of cirrhosis-related brain impairment is complex, and many other factors, such as the accumulation of manganese (36) and malnutrition, may also contribute to GMV loss. Therefore, further studies are needed to consider these factors and perform multiple factor analysis. Finally, even though we had specific hypotheses based on previous studies, we did not correct for multiple comparisons in the correlation analysis between GMV, serum MDA and plasma ammonia in cirrhotic patients. Stricter thresholds should be used in future studies.
In conclusion, the present study showed that increased serum MDA levels were associated with GMV loss in patients with non-alcoholic cirrhosis, suggesting that OS may be involved in GMV loss in cirrhosis. Further studies are needed to determine whether OS is causally related to GMV loss in cirrhosis. If confirmed, treatments that decrease OS may help prevent the irreversible neurocognitive impairment caused by GMV loss in cirrhotic patients.
Acknowledgements
Funding: This work was supported by the Natural Science Foundation of Hunan Province (2016JJ6149) and the National Natural Science Foundation of China (81471715).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Medical Research Ethics Committee of the Third Xiangya Hospital of Central South University. Written informed consent was obtained from all participants before the study.
References
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology 2009;50:2014-21. [Crossref] [PubMed]
- Guevara M, Baccaro ME, Gómez-Ansón B, Frisoni G, Testa C, Torre A, Molinuevo JL, Rami L, Pereira G, Sotil EU, Córdoba J, Arroyo V, Ginès P. Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J Hepatol 2011;55:564-73. [Crossref] [PubMed]
- Tarter RE, Hays AL, Sandford SS, Van Thiel DH. Cerebral morphological abnormalities associated with non-alcoholic cirrhosis. Lancet 1986;2:893-5. [Crossref] [PubMed]
- Qi R, Zhang LJ, Zhong J, Zhu T, Zhang Z, Xu C, Zheng G, Lu GM. Grey and white matter abnormalities in minimal hepatic encephalopathy: a study combining voxel-based morphometry and tract-based spatial statistics. Eur Radiol 2013;23:3370-8. [Crossref] [PubMed]
- Zhang LJ, Qi R, Zhong J, Xu Q, Zheng G, Lu GM. The effect of hepatic encephalopathy, hepatic failure, and portosystemic shunt on brain volume of cirrhotic patients: a voxel-based morphometry study. PLoS One 2012;7:e42824. [Crossref] [PubMed]
- Chen HJ, Zhu XQ, Shu H, Yang M, Zhang Y, Ding J, Wang Y, Teng GJ. Structural and functional cerebral impairments in cirrhotic patients with a history of overt hepatic encephalopathy. Eur J Radiol 2012;81:2463-9. [Crossref] [PubMed]
- Butterworth R. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007;22:309-20. [Crossref] [PubMed]
- Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem 2017;86:715-48. [Crossref] [PubMed]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239-47. [Crossref] [PubMed]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 2006;97:1634-58. [Crossref] [PubMed]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci 2004;101:2070-5. [Crossref] [PubMed]
- Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc Natl Acad Sci 2013;110:7904-9. [Crossref] [PubMed]
- Kallianpur KJ, Gerschenson M, Mitchell BI. LiButti DE, Umaki TM, Ndhlovu LC, Nakamoto BK, Chow DC, Shikuma CM. Oxidative mitochondrial DNA damage in peripheral blood mononuclear cells is associated with reduced volumes of hippocampus and subcortical gray matter in chronically HIV-infected patients. Mitochondrion 2016;28:8-15. [Crossref] [PubMed]
- Moran C, Münch G, Forbes JM, Beare R, Blizzard L, Venn AJ, Phan TG, Chen J, Srikanth V. Type 2 diabetes, skin autofluorescence, and brain atrophy. Diabetes 2015;64:279-83. [Crossref] [PubMed]
- Fraguas D, Gonzalez-Pinto A, Micó JA, Reig S, Parellada M, Martínez-Cengotitabengoa M, Castro-Fornieles J, Rapado-Castro M, Baeza I, Janssen J, Desco M, Leza JC, Arango C. Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr Res 2012;137:58-65. [Crossref] [PubMed]
- Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun 1998;247:166-70. [Crossref] [PubMed]
- Ljubuncic P, Tanne Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut 2000;47:710-6. [Crossref] [PubMed]
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 1995;41:1819-28. [PubMed]
- Lee KC, Yang YY, Wang YW, Lee FY, Loong CC, Hou MC, Lin HC, Lee SD. Increased plasma malondialdehyde in patients with viral cirrhosis and its relationships to plasma nitric oxide, endotoxin, and portal pressure. Dig Dis Sci 2010;55:2077-85. [Crossref] [PubMed]
- Galicia-Moreno M, Rosique-Oramas D, Medina-Avila Z, Álvarez-Torres T, Falcón D, Higuera-de la Tijera F, Béjar YL, Cordero-Pérez P, Muñoz-Espinosa L, Pérez-Hernández JL, Kershenobich D, Gutierrez-Reyes G. Behavior of oxidative stress markers in alcoholic liver cirrhosis patients. Oxid Med Cell Longev 2016;2016:9370565. [Crossref] [PubMed]
- Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta 2003;1637:142-50. [Crossref] [PubMed]
- Jayakumar AR, Norenberg MD. Oxidative Stress in Hepatic Encephalopathy. In: Mullen K, Prakash R. editors. Hepatic Encephalopathy. Clinical Gastroenterology. Totowa, NJ: Humana Press, 2012;47-70
- Sutherland GT, Sheedy D, Kril JJ. Neuropathology of alcoholism. Handb Clin Neurol 2014;125:603-15. [Crossref] [PubMed]
- Farokhian F, Yang C, Beheshti I, Matsuda H, Wu S. Age-related gray and white matter changes in normal adult brains. Aging Dis 2017;8:899-909. [Crossref] [PubMed]
- Shi L, Du FL, Sun ZW, Zhang L, Chen YY, Xie TM, Li PJ, Huang S, Dong BQ, Zhang MM. Radiation-induced gray matter atrophy in patients with nasopharyngeal carcinoma after intensity modulated radiotherapy: a MRI magnetic resonance imaging voxel-based morphometry study. Quant Imaging Med Surg 2018;8:902-09. [Crossref] [PubMed]
- Su H, Zuo C, Zhang H, Jiao F, Zhang B, Tang W, Geng D, Guan Y, Shi S. Regional cerebral metabolism alterations affect resting-state functional connectivity in major depressive disorder. Quant Imaging Med Surg. 2018;8:910-24. [Crossref] [PubMed]
- Poveda MJ, Bernabeu A, Concepción L, Roa E, de Madaria E, Zapater P, Pérez-Mateo M, Jover R. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010;52:481-7. [Crossref] [PubMed]
- Meng LP, Chen YC, Li YH, Zhu JS, Ye JL. Viability assessment of magnetic resonance spectroscopy for the detection of minimal hepatic encephalopathy severity. Eur J Radiol 2015;84:2019-23. [Crossref] [PubMed]
- Yang X, Bosoi CR, Jiang W, Tremblay M, Rose CF. Portacaval anastomosis-induced hyperammonemia does not lead to oxidative stress. Metab Brain Dis 2010;25:11-5. [Crossref] [PubMed]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med 2004;10 Suppl:S18-25. [Crossref] [PubMed]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem 1997;68:2092-7. [Crossref] [PubMed]
- Montoliu C, Cauli O, Urios A, ElMlili N, Serra MA, Giner-Duran R, González-Lopez O, Del Olmo JA, Wassel A, Rodrigo JM, Felipo V. 3-nitro-tyrosine as a peripheral biomarker of minimal hepatic encephalopathy in patients with liver cirrhosis. Am J Gastroenterol 2011;106:1629-37. [Crossref] [PubMed]
- Gimenez-Garzó C, Urios A, Agustí A, González-López O, Escudero-García D, Escudero-Sanchis A, Serra MA, Giner-Durán R, Montoliu C, Felipo V. Is cognitive impairment in cirrhotic patients due to increased peroxynitrite and oxidative stress? Antioxid Redox Signal 2015;22:871-7. [Crossref] [PubMed]
- Kundra A, Jain A, Banga A, Bajaj G, Kar P. Evaluation of plasma ammonia levels in patients with acute liver failure and chronic liver disease and its correlation with the severity of hepatic encephalopathy and clinical features of raised intracranial tension. Clin Biochem 2005;38:696-9. [Crossref] [PubMed]
- Yang L, Omori K, Omori K, Otani H, Suzukawa J, Inagaki C. GABAC receptor agonist suppressed ammonia-induced apoptosis in cultured rat hippocampal neurons by restoring phosphorylated BAD level. J Neurochem 2003;87:791-800. [Crossref] [PubMed]
- Butterworth RF, Spahr L, Fontaine S, Layrargues GP. Manganese toxicity, dopaminergic dysfunction and hepatic encephalopathy. Metab Brain Dis 1995;10:259-67. [Crossref] [PubMed]


