
Tumor enhancement of colorectal liver metastases on preoperative gadobutrol-enhanced MRI at 5 minutes post-contrast injection is associated with overall survival post-hepatectomy
Introduction
Colorectal cancer is the second leading cause of cancer deaths (after lung) in the United States (1). Liver metastases develop in approximately 50% of patients with colorectal cancer despite screening programs and early treatment (2). With new advances in surgery and chemotherapy, patients who meet surgical resectability criteria have significantly improved morbidity and mortality with 5- and 10-year survival rates of 38% and 26% respectively (3,4).
It is increasingly being understood that colorectal cancer is not a homogeneous disease and that the biology of tumors may account for a large range of differences in response to treatment (5). Preoperative imaging may be a simple, noninvasive way of risk-stratifying patients prior to treatment.
A recently published study demonstrated that late gadolinium enhancement of colorectal liver metastases (CRLM) on preoperative gadobutrol-enhanced MRI is associated with overall survival post-hepatectomy (6). In that study, the authors measured late gadolinium enhancement using the “target tumor enhancement” (TTE), which they defined as the mean liver-lesion contrast-to-noise ratio (CNR) of the two largest tumors on 10-minute delayed phase (6). However, many standard liver MRI protocols have now reduced the delayed phase imaging time to 5-minutes in order to improve workflow (7). The goal of this study was to determine whether TTE measured at 5-minute delayed phase is also associated with survival.
Methods
This was a single-institutional, retrospective study, which was approved by the institutional research ethics board.
The patient population included all patients with colorectal cancer liver metastases (CRCLM) who had received a clinical gadobutrol-enhanced MRI after treatment with chemotherapy and prior to liver surgery between January 1, 2006 and December 31, 2012 for diagnosis and/or staging. We excluded patients who did not have 5-minute delayed phase imaging or where MRI image quality was unacceptable for analysis. Patients who did not have any tumors that met criteria for measurable target lesions (>10 mm) were also excluded. Any patients who died within 30 days of surgery were also excluded from the analysis in order to exclude perioperative mortality.
As per standard clinical protocols at our institution, patients received contrast-enhanced liver MRI with gadobutrol on either a 1.5T (GE Twinspeed™) or 3.0 T (Philips Achieva™) MRI scanner with an eight-channel body phased array coil covering the entire liver. Delayed phase, contrast-enhanced, 3D axial T1 imaging was performed at 5-minute post-contrast (protocol at 1.5T: TR ~4.5, TE ~2.2, flip angle ~15, slice thickness =5 mm, spacing =2.5 mm, FOV =380 mm, matrix =320×192. Protocol at 3.0T: TR ~3.0, TE ~1.4, flip angle ~10, slice thickness =3 mm, spacing =1.5 mm, FOV ~380, matrix ~250×250).
We measured the TTE at 5-minute delayed phase for all patients using methods previously described (6). Up to two target lesions measuring at least 10 mm in long axis diameter were chosen for analysis. The axial slice at which the tumor was largest was chosen. A round ROI was drawn to most closely approximate the entire tumor in order to determine the signal intensity of the tumor. Five 1–2 cm round ROIs were drawn on the adjacent background liver parenchyma (taking care to avoid major blood vessels) in order to determine the mean signal intensity of the background liver. The standard deviation of the background noise was calculated by taking the mean standard deviation of eight 1–2 cm ROIs drawn in the background noise in each of the four quadrants. The CNR of up to two target lesions on 5 minute delayed phase was measured and the mean CNR of up to two target lesions was taken as the TTE. The lesion-liver CNR was measured as follows:
All imaging analysis was performed on standard, clinical PACS software used at our institution (Agfa Impax 6.3.1, AGFA HealthCare N.V., Belgium). Imaging analysis was performed by a single reader (Helen M. C. Cheung, 6 years of experience) blinded to all clinical information other than the history of CRLM.
The primary clinical end-point was overall survival. The following additional clinical information was also obtained: age, sex, number of liver tumors, size of largest liver tumor, time from diagnosis of primary colorectal cancer to time to diagnosis of hepatic metastases, number of positive regional lymph nodes seen on pathology in the primary colorectal cancer, and preoperative carcinoembryonic antigen level. Based on our clinical data, we calculated the patient’s clinical risk score, which is a 5-point score that has been validated as a predictor of long-term, postoperative survival and is commonly used to risk-stratify patients for surgery (8).
Statistical analysis
Patients were dichotomized into strong and weak TTE using the Youden index for three-year survival (9). The association between TTE at 5 minutes and overall survival was determined using univariate analysis using Kaplan-Meier statistics. The association between TTE at 5 minutes and overall survival was determined using multivariate analysis using Cox-regression analysis, adjusted for clinical risk score.
Additional descriptive analysis was performed on demographic variables (age and sex). Chi-Square tests were used in order to determine if there were differences in demographic data between strong and weak TTE groups.
All analyses were performed on SPSS (SPSS Statistics for Macintosh, Version 24.0, 2016. IBM Corp., Armonk, NY, USA).
Results
There were a total of 121 patients who met inclusion/exclusion criteria for the study. There were 70 (58%) men and 51 (42%) women in our cohort. The mean age was 63 years. Demographic data is shown in Table 1. There was no significant difference in demographic variables between strong and weak TTE groups. There was no difference in the proportion of patients with weak or strong TTE between 1.5T and 3.0T (P=0.104).
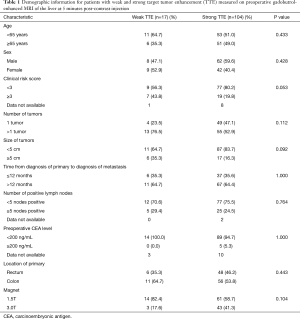
Full table
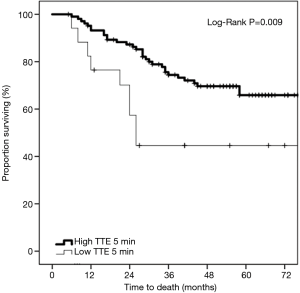
Based on the Youden index, patients were dichotomized into strong and weak TTE at a threshold of −26. There were 17 patients (with 9 deaths) with weak TTE and 104 patients (with 31 deaths) with strong TTE (Figure 1).
On univariate Kaplan-Meier analysis, there was a significant difference in survival between the strong and weak TTE groups (Figure 2) (log-rank P=0.009). At 36 months, 74.4% were surviving in the strong TTE group compared to only 44.6% in the weak TTE group.
On multivariable Cox-regression analysis, there was a significant difference in survival between the strong and weak TTE groups (P=0.026), even after adjusting for clinical risk score. The adjusted hazard ratio of death for patients with low TTE was 0.40 (95% CI: 0.18–0.90) (Table 2).
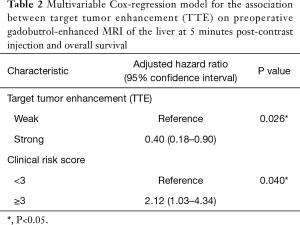
Full table
Discussion
This study demonstrates that TTE of chemotherapy-treated CRLM at 5 minutes post-contrast injection with gadobutrol is associated with overall survival post-hepatectomy. The proportion of patients surviving at 36 months with strongly enhancing tumors was 74.4% compared to only 44.6% with weakly enhancing tumors.
A previous study demonstrated that TTE of chemotherapy-treated CRLM at 10 minutes post-contrast injection with gadobutrol is associated with tumor fibrosis and overall survival post-hepatectomy (6). However, we note that the proportion of patients who were in the low vs. high TTE group at 5 minutes in the current study is different than the proportion of patients who were in the low vs. high TTE group at 10 minutes in the previous study despite being similar populations (6). In the current study, only 14% of patients were in the low TTE group at 5 minutes, whereas 61% of patients were in the low TTE group at 10 minutes in the prior study (6). This suggests that the mechanism of TTE may be different at 5 versus 10 minutes. Prior studies in the cardiac MRI literature has shown that fibrosis is best observed between 10–30 minutes (10). Therefore, although high TTE at 10 minutes was correlated with tumor fibrosis, high TTE at 5 minutes may represent a different mechanism.
The mechanism by which TTE at 5 minutes is associated with survival is unclear based on this study. Possible mechanisms may include differences in vascularity and/or perfusion between high and low TTE groups. Prior studies using dynamic contrast enhanced MRI (DCE-MRI) have shown variability in tumor vessel volume and perfusion volume, which may be related to response to chemotherapy (11-13). Further prospective studies with high-resolution matched radiologic-pathologic correlation are required in order to determine whether tumor vascularity or other histopathological variables may be associated with TTE at 5 minutes.
There are a number of additional limitations to this study. This study represents a single-institution, retrospective study. Further prospective, multi-center studies are required for external validation. Due to its retrospective nature, there are a number of clinical and technical confounders. In particular, we were unable to control for chemotherapy regimens used in this study. All patients received standard-of-care chemotherapy regimens as determined by their clinical team. The chemotherapy regimens received by the patients were not standardized and different patients received different chemotherapy regimens. This could be a potential confounder. Future studies are required in order to control for this and to better understand the role of chemotherapy on TTE.
Technical confounders such as magnetic field strength and phased array surface coil may also affect CNR measurements (14,15). Future studies are also required in order to optimize measurement techniques for TTE, including measuring signal intensity increase from pre-contrast to delayed phase or signal intensity involving the entire volume of tumor rather than a single axial slice.
In conclusion, this study provides preliminary evidence that tumor enhancement of CRLM at 5 minutes post-contrast injection on gadobutrol-enhanced MRI may provide preoperative prognostic information. This may potentially be helpful for risk stratification of patients for surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf
- Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405-10. [Crossref] [PubMed]
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283-301. [PubMed]
- Collins D, Chua H. Contemporary surgical management of synchronous colorectal liver metastases. F1000Res 2017;6:598. [Crossref] [PubMed]
- Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol 2017;14:235-46. [Crossref] [PubMed]
- Cheung HMC, Karanicolas PJ, Hsieh E, Coburn N, Maraj T, Kim JK, Elhakim H, Haider MA, Law C, Milot L. Late gadolinium enhancement of colorectal liver metastases post-chemotherapy is associated with tumour fibrosis and overall survival post-hepatectomy. Eur Radiol 2018;28:3505-12. [Crossref] [PubMed]
- Donato H, Franca M, Candelaria I, Caseiro-Alves F. Liver MRI: From basic protocol to advanced techniques. Eur J Radiol 2017;93:30-9. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph-Edwards A, Wintersperger BJ, Crean A. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging 2013;6:587-96. [Crossref] [PubMed]
- Kannan P, Kretzschmar WW, Winter H, Warren DR, Bates R, Allen PD, Syed N, Irving B, Papiez BW, Kaeppler J, Markelc B, Kinchesh P, Gilchrist S, Smart SC, Schnabel JA, Maughan TS, Harris AL, Muschel RJ, Partridge M, Sharma RA, Kersemans V. Functional parameters derived from magnetic resonance imaging reflect vascular morphology in preclinical tumors and in human liver metastases. Clin Cancer Res 2018;24:4694-704. [Crossref] [PubMed]
- Tampellini M, Gned D, Baratelli C, Brizzi MP, Ottone A, Alabiso I, Bertaggia C, Di Maio M, Scagliotti GV, Veltri A. Changes in hepatic perfusion assessed by dynamic contrast enhanced MRI, associated with morphologic evaluation, in patients with liver metastases from colorectal cancer treated with first-line chemotherapy. Radiol Med 2016;121:950-7. [Crossref] [PubMed]
- Kim JH, Joo I, Kim TY, Han SW, Kim YJ, Lee JM, Han JK. Diffusion-Related MRI Parameters for Assessing Early Treatment Response of Liver Metastases to Cytotoxic Therapy in Colorectal Cancer. AJR Am J Roentgenol 2016;207:W26-32. [Crossref] [PubMed]
- Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, Li X, Runge VM. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol 2015;50:330-8. [Crossref] [PubMed]
- Xu L, Gu S, Feng Q, Liang C, Xin SX. Quantitative study of liver magnetic resonance spectroscopy quality at 3T using body and phased array coils with physical analysis and clinical evaluation. PLoS One 2015;10:e0122999. [Crossref] [PubMed]


