Bone marrow reconversion mimicking pelvis metastases in a patient with rectal cancer: a pitfall on magnetic resonance images
Introduction
The bone marrow is one of the largest organs in the body. There are two main types of bone marrow in human body: red and yellow bone marrow in the human body. Their proportions are determined by the overall distribution of fat and water, which ultimately determines the magnetic resonance imaging (MRI) signal intensity. At birth, red bone marrow (40% fat, 40% water and 20% protein) is present throughout the entire skeleton. It gradually converts into yellow bone marrow (80% fat, 15% water and 5% protein) (1). The conversion begins in the extremities and progresses toward the axial skeleton, and it can always be completed by approximately 25 years of age (1-3).
When the existing hematopoietic marrow cannot meet the demand for hematopoiesis, the body shifts marrow distribution with replacement of yellow marrow by red marrow, which is called bone marrow reconversion (BMR) (4). However, the occurrence of BMR can be misleading, and its interpretation of imaging can be challenging. Regarding the patients with malignancies, it is occasionally difficult to differentiate BMR from bone metastasis, raising the risk of delayed or missed diagnoses. BMR located in some vertebrae of patients with malignant tumor mimicking bone metastases has been reported by sporadic literatures (5-8). However, little literature reports BMR located in the pelvis. Herein, we report a case of BMR in a patient with rectal cancer which was misdiagnosed as bone metastases by a radiologist using original pelvic MRI.
Case presentation
A 45-year-old woman (height 150 cm, body weight 40 kg) was referred to our hospital for treatment of bloody stools, which lasted for about 3 months. In addition, she had a 2-year history of constipation, and roughly a 2-year history of grade 2 anemia (Hb: 60–90 g/L). A digital rectal examination (DRE) revealed a tumor about 6 cm from the anus, which was firm, immobile and it occupied 2/3 of the intestinal tract lumen. Meanwhile, laboratory examination revealed anemia (Hb: 80 g/L).
The patient subsequently underwent a pelvic MRI on April 1st, 2017. The pelvic MR images demonstrated a primary tumor located on upper rectum and penetrated the surface of the visceral peritoneum with an inhomogeneous hypointense signal on T1-weighted images and inhomogeneous hyperintense signal on T2- and diffusion-weighted images. Multiple enlarged lymph nodes were identified on the pelvic region (regional) and the peritoneal region (nonregional) with hypointense signals on T1-weighted images, and hyperintense signals on T2- and diffusion-weighted images. Diffuse abnormal signals were noticed in the sacrum and bilateral ilia with hypointense signals on T1- and T2-weighted images, hyperintense signals on diffusion-weighted images, and diffuse mild enhancement on fat-saturated T1-weighted images (Figure 1). Based on the MRI features of this patient, the radiologist diagnosed the patient as rectal cancer with multiple bone metastases of the sacrum and bilateral ilia, staged as T4N2M1.
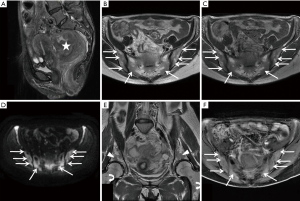
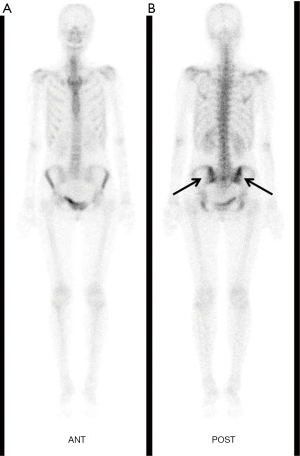
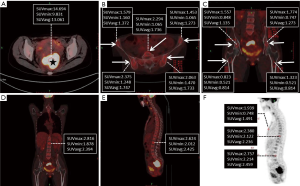
Consequently, colonoscopy examination was performed which indicated that the tumor was located about 6 cm away from the anus. Pathological examination of the tissue showed evidence of rectal adenocarcinoma. For further evaluation, whole body bone scintigraphy with 99mTc-methylene diphosphonate (99mTc-MDP) was performed on April 6th, and revealed abnormal radiotracer uptake in the pelvis, especially in sacroiliac joint, which suggested metastatic disease (Figure 2). In addition, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (18F-FDG PET/CT) was performed on April 11th, and revealed obvious 18F-FDG uptake in the primary tumor with maximum standardized uptake value (SUVmax) of 14.69, while there were slight diffuse 18F-FDG uptakes in the vertebras, sternum, sacrum, ilium, and femurs with a SUVmax of 2.816 (Figure 3). The 18F-FDG PET/CT findings: (I) diffuse distribution in the vertebral body, sternum, sacrum, ilium and femurs; (II) slightly high uptake with lower SUVmax, contributed to the diagnosis of BMR. Ultimately, the diagnosis of BMR was histopathologically confirmed by a bone marrow biopsy of the ilium, which was performed on April 12th and revealed erythroid hyperplasia rather than bone marrow metastasis.
Discussion
Generally, in a healthy person, the bone marrow undergoes conversion from hematopoietically active red marrow to hematopoietically inactive yellow marrow in a very orderly and predictable pattern (9). This conversion is from distal to proximal, from the appendicular to the axial skeleton and from the diaphyses of the long bones toward the metaphyses (9). Increased demand for hematopoiesis prompts reconversion from fatty marrow to active red marrow, and the reconversion process occurs in the exact reverse order: centripetally from the axial to the appendicular skeleton (2). BMR can be a consequence of both non-medical conditions (e.g., heavy smoking and intense oxygen-bearing sports) and medical conditions (e.g., obesity and related respiratory disorders, diabetes, chronic conditions related to anemia, treated with hematopoietic growth factors) (4). In our case, since the primary tumor in the upper rectum was relatively large, and the patient had a 2-year history of constipation and severe anemia, we deemed that rectal cancer had developed over the past 2 years. In addition, during the 2-year history of anemia, the patient did not receive medical treatment for anemia and she just took food supplements. In this report, we described the rectal cancer patient with pelvic MRI features mimicking pelvis metastases due to BMR, which was attributed to chronic anemia.
MRI is the most sensitive imaging modality that evaluates the bone marrow. The signal intensity depends on the relative amount of protein, water, fat, and cells within the marrow (10). Yellow marrow appears hyperintense on T1-weighted images, because it is composed of fatty elements, and it shows intermediate response to high signal intensity on T2-weighted images. It saturates similarly to subcutaneous fat on T2-weighted sequences with fat saturation and on STIR sequences. Red marrow demonstrates low/intermediate signal intensity on T1-weighted images, and it shows intermediate signal intensity on T2-weighted images, which can result in difficulty in distinguishing between red marrow and yellow marrow; it displays mild high signal intensity on STIR images.
Although MRI is an excellent noninvasive modality for evaluating bone marrow and detecting marrow lesions, it causes a dilemma when differentiating physiological marrow reconversion from suspicious pathologies. In patients with malignancies, it is occasionally difficult to differentiate BMR from bone metastasis. Sporadic literatures reported BMR located in vertebrae (5-8). For example, Okuda et al. reported the MRI of a patient with esophageal adenocarcinoma showed patchy signal changes in the thoracic vertebral body, which mimicked bone metastases on MR (5). Tanaka reported the MRI of a patient with esophageal carcinoma showed patchy signal changes in the lumbar vertebral body, which mimicked bone metastases (6). Yu and colleagues reported the MRI of a patient with prostate cancer showed patchy signal changes in the lumbar vertebral body, which mimicked bone metastases (7). In addition, several literatures reported BMR induced by granulocyte-colony stimulating factor (G-CSF) located in the lower extremities mimicking bone metastases on MRI (11,12). However, there has been little literature reporting the BMR was diffusely located in pelvis. In our case, the MR images demonstrated a diffuse abnormal signal in the sacrum and bilateral ilia with hypointense signals on T1- and T2-weighted images, hyperintense signals on diffusion-weighted images and diffuse mild enhancement on fat-saturated T1-weighted images. It is often difficult to distinguish bone metastasis and BMR with conventional MRI. The MRI chemical shift technique assesses fatty infiltration, which suggests yellow bone marrow, and distinguishes between BMR and bone metastasis (5). However, MRI chemical shift techniques, for instance, the proton density fat fraction (PDFF) technique, is commonly used for hepatic MRI rather than pelvic MRI for clinical purposes.
A bone scan or bone scintigraphy can help diagnose the whole body. Bone scintigraphy is generally used clinically to evaluate a number of bone conditions, including cancer of the bone or metastasis, locate bone inflammation or fractures, as well as bone infections (13). The most common radiopharmaceutical for bone scintigraphy is 99mTc with MDP, which adsorbs onto the crystalline hydroxyapatite mineral of the bone (13). In our case, the whole-body bone scintigraphy with 99mTc-MDP revealed suspicious bone metastases in the pelvis. Therefore, whole body bone scintigraphy with 99mTc-MDP could not differentiate the BMR from bone metastases. It is reported that imaging of 111In-Cl3 scintigraphy is effective when differentiating BMR from bone metastases (5,8). 111In-Cl3 scintigraphy is a noninvasive technique to evaluate the anatomic extent of the erythropoietic element, because of its transportation in the plasma by transferrin and its suitable energy characteristics (5). However, 111In-Cl3 scintigraphy is not commonly used clinically, especially in developing countries.
PET/CT scanning, with the tracer 18F-FDG, called 18F-FDG PET/CT, is widely used in clinical oncology for diagnosis, staging, and monitoring of the treatment of cancers. However, several literatures have reported BMR that was confused with metastasis when using 18F-FDG PET/CT (6,8). Meanwhile, a previous report evaluating imaging findings of hyperplastic hematopoietic bone marrow and bone metastasis showed that if the SUVmax of a bone lesion was more than 3.6 on 18F-FDG PET/CT, the lesion could be considered as metastatic (14). In our case, the SUVmax of the vertebrae, sternum and pelvis were all lower than 3.6. Meanwhile, the symmetric distribution pattern of slight high uptakes in the vertebras, sternum, sacrum, ilia and femurs contributed to the correct diagnosis of BMR. In addition, the uptake of 18F-FDG by tissues reveals the degree of glucose uptake, rather than the Hb level. Therefore, in our case, the anemia condition may not directly affect the SUVmax of the tissues.
In summary, we encountered a rectal cancer patient with BMR due to chronic anemia, and the pelvic MRI demonstrated bone marrow signal changes were related to reconversion from fatty to hematopoietic marrow, mimicking pelvic bone metastasis. Combined with the features of bone scintigraphy, we also could not rule out the possibility of pelvic metastasis. In our case, its 18F-FDG PET/CT features were: (I) slight high uptake with lower than SUVmax of 3.6; (II) diffused distribution in the vertebras, sternum, sacrum, ilium and femurs. These features highly contributed to the diagnosis of BMR rather than bone metastasis. Therefore, radiologists and oncologists should be aware of the possible appearance of BMR mimicking bone metastasis on pelvic MR images to avoid misinterpretation and excessive treatment.
Acknowledgements
Funding: This study was funded by grants from the Zhejiang Provincial Natural Science Foundation of China [grant number: LY17H180001], the health and family planning commission of Zhejiang Province (grant number: 2018KY063) and Chinese Medicine Research Program of Zhejiang Province (grant number: 2018ZZ014).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Guillerman RP. Marrow: red, yellow and bad. Pediatr Radiol 2013;43 Suppl 1:S181-92. [Crossref] [PubMed]
- Chan BY, Gill KG, Rebsamen SL, Nguyen JC. MR Imaging of Pediatric Bone Marrow. Radiographics 2016;36:1911-30. [Crossref] [PubMed]
- Ollivier L, Gerber S, Vanel D, Brisse H, Leclère J. Improving the interpretation of bone marrow imaging in cancer patients. Cancer Imaging 2006;6:194-8. [Crossref] [PubMed]
- Małkiewicz A, Dziedzic M. Bone marrow reconversion - imaging of physiological changes in bone marrow. Pol J Radiol 2012;77:45-50. [PubMed]
- Okuda S, Nishibori H, Hoshi H. Utility of 111In-Cl3 Scintigraphy for Differentiating Between Bone Marrow Reconversion and Bone Metastasis with Esophageal Adenocarcinoma. J Nucl Med Technol 2018;46:63-4. [Crossref]
- Tanaka T, Gobara H, Inai R, Iguchi T, Tada A, Sato S, Yanai H, Kanazawa S. A Case of Focal Bone Marrow Reconversion Mimicking Bone Metastasis: The Value of 111Indium Chloride. Acta Med Okayama 2016;70:285-9. [PubMed]
- Yu YS, Li WH, Li MH, Meng X, Kong LI, Yu JM. False-positive diagnosis of disease progression by magnetic resonance imaging for response assessment in prostate cancer with bone metastases: A case report and review of the pitfalls of images in the literature. Oncol Lett 2015;10:3585-90. [Crossref] [PubMed]
- Okuyama C, Sasaki N, Nishimura M, Matsushima S, Yoshimatsu R. Active Bone Marrow With Focal FDG Accumulation Mimicking Bone Metastasis With a Case of Early Esophageal Cancer. Clin Nucl Med 2018;43:258-61. [PubMed]
- Andrews CL. From the RSNA Refresher Courses. Radiological Society of North America. Evaluation of the marrow space in the adult hip. Radiographics. 2000;20:S27-42. [Crossref] [PubMed]
- Caranci F, Tedeschi E, Ugga L, D'Amico A, Schipani S, Bartollino S, Russo C, Splendiani A, Briganti F, Zappia M, Melone MAB, Masciocchi C, Brunese L. Magnetic Resonance Imaging correlates of benign and malignant alterations of the spinal bone marrow. Acta Biomed 2018;89:18-33. [PubMed]
- Cakir FB, Baysal B, Dogan O. False positivity of magnetic resonance imaging under the effect of granulocyte-colony stimulating factor in a child with leukemia. Contemp Oncol (Pozn) 2013;17:334-6. [Crossref] [PubMed]
- Naples JC, Taljanovic MS, Graham ML, Hunter TB. Granulocyte-Stimulating Factor-Induced Bone Marrow Reconversion Simulating Neuroblastoma Metastases on MRI: Case Report and Literature Review. Radiol Case Rep 2016;2:5-9. [Crossref] [PubMed]
- Mallee WH, Wang J, Poolman RW, Kloen P, Maas M, de Vet HC, Doornberg JN. Computed tomography versus magnetic resonance imaging versus bone scintigraphy for clinically suspected scaphoid fractures in patients with negative plain radiographs. Cochrane Database Syst Rev 2015. [PubMed]
- Shigematsu Y, Hirai T, Kawanaka K, Shiraishi S, Yoshida M, Kitajima M, Uetani H, Azuma M, Iryo Y, Yamashita Y. Distinguishing imaging features between spinal hyperplastic hematopoietic bone marrow and bone metastasis. AJNR Am J Neuroradiol 2014;35:2013-20. [Crossref] [PubMed]


