Differential diagnosis value of the ultrasound gray scale ratio for papillary thyroid microcarcinomas and micronodular goiters
Introduction
Ultrasound echo intensity is a commonly used parameter to evaluate thyroid nodules. The echo intensity ranges from weak to strong, and manifests as a gray scale from black to white in the sonogram. At present, the echo intensity of thyroid nodules is often subjectively determined by an observer. Based on a gray scale, from black to white, the echo intensity is divided into five grades comprising the following: no echo, extremely low echo (lower than neck strap muscle), low echo (between the neck strap muscle and the thyroid), equal echo (consistent with the thyroid), and high echo (higher than the thyroid) (1-3). There is no clear boundary between any two adjacent grades, which may lead to significant deviation among different observers and may reduce the application value of echo intensity in the diagnosis of thyroid nodules. A direct or indirect objective numerical quantification of a gray scale value is likely to be more accurate than an observer’s naked-eye determination. In addition, the technology of computer intelligence assisted diagnosis has become increasingly more advanced, and gray scale quantification may help rapid and accurate determination by computer intelligence assisted diagnosis.
In 2013, Erol et al. (4) proposed the lesion echogenicity ratio (LER) for the differential diagnosis of benign and malignant breast lesions, and gray scale values of a lesion and the surrounding fat lobules were obtained using a gray scale histogram, and the ratio of the latter to the former constituted the LER. In their study, the LER of malignant lesions was 3.10±0.87, which was significantly higher than 1.63±0.41 for the benign lesions. However, the application of LER to differentiate benign and malignant breast lesions has some limitations, particularly regarding the menstrual cycle, age, and other related physiological factors. In addition, due to the scattered distribution of the lesion-adjacent fat tissue, the measurement results obtained from different regions can be different. Given thyroid tissue is not the same as breast tissue, the LER of the thyroid may be less affected in respect of the above factors. The surrounding normal or relatively normal glands can be used as control areas and deviation among different observers is likely reduced. LER results from thyroid lesions are likely to be obtained more objectively. In this study, to obtain a positively correlated index with the echo intensity of thyroid lesions, we adopted the ultrasound gray scale ratio (UGSR), that is, the ratio of a lesions’ gray scale value to that of the surrounding normal thyroid tissue. The objective was to provide a new method to quantify the echo intensity of thyroid lesions.
Methods
Participants
This is a retrospective analysis, we identified a total of 2,984 consecutive patients with thyroid nodules treated in the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, from December 2012 to December 2016. We excluded thyroid nodules with a diameter >1.0 or <0.5 cm, or nodules complicated with Hashimoto’s thyroiditis, cystic-dominated nodules (where the cystic component was >50% of the nodule volume) (3,5,6), or calcification-dominated nodules (where nodules could not be measured due to obvious calcification). Finally, 926 patients with 1,056 thyroid nodules who met the inclusion criteria were included in the study. There were 185 male and 741 female participants. The mean age was 49±11 years. Figure 1 is a flow chart showing the characteristics of the study participants. This study was approved by the ethics committee of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. Written informed consent was waived due to the non-interventional and retrospective nature of this study.
Ultrasonic examination
Ultrasonic examination of thyroid lesions was performed using one of the following ultrasonic diagnostic scanners: MyLab 70 XVG (Genova, Italy), Esaote MyLab Classic C (Genova, Italy), Esaote Mylab 90 (Genova, Italy), and so on (Table 1). Then, 5–10 MHz broadband linear array probes were used for this study, and the central frequency was 7.5 MHz. The patients were placed in a supine position, with the head positioned as far back as possible to expose the anterior region of the neck. Ultrasonic scanning of the lesions was performed in longitudinal, transverse, and oblique sections. The thyroid nodule number, size, shape, boundary, surrounding acoustic halo, internal echo, calcification, internal and peripheral blood supply, and bilateral neck lymph nodes were examined.
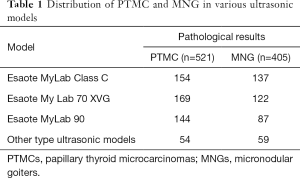
Full table
Image analysis
Ultrasound data selected from the picture archiving and communication systems (PACS) were independently analyzed by two senior imaging specialists with 15 years of experience who were blinded to the pathological results. The region of interest (ROI), size, and measurement area of thyroid nodules and the surrounding normal thyroid tissue, were determined. The gray values of papillary thyroid microcarcinomas (PTMCs), micronodular goiters (MNGs) and their surrounding normal thyroid tissue were measured using gray histogram software from the RADinfo reading system (Zhejiang RAD Information Technology Co., Ltd., China). In ultrasonic transverse scanning, nodules in the upper and lower poles of the thyroid often lack normal thyroid tissue as a control. Therefore, the longitudinal ultrasonic images were adopted in this study. When measuring the gray scale of nodules with homogeneous echo (Figures 2,3), a ROI >1/2 the nodule area was adopted. For nodules with heterogeneous echo (Figure 4), the echo region with the largest area proportion was selected, and a ROI >1/2 the nodule area was adopted. When gray scale of the surrounding normal thyroid tissue was measured, the surrounding areas at the same level with the ROI of the measured nodules were selected to acquire the data for analysis. Finally, the UGSR was calculated.
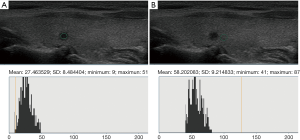
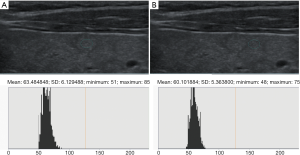
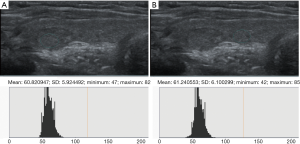
Statistical analysis
All statistical analyses were undertaken using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). The comparison between the two groups was conducted using the Mann-Whitney test. Using sensitivity as the ordinate and specificity as the abscissa, the receiver operating characteristic (ROC) curve for the UGSR to PTMCs and MNGs was plotted. The area under the curve (AUC) was calculated. The optimal threshold of the UGSR was determined comparing the Youden index, sensitivity, specificity, and other diagnostic parameters.
Results
In 561 PTMCs, the mean UGSR was 0.54 (SD: 0.16; range: 0.24–1.26). In 515 MNGs, the mean UGSR was 0.87 (SD: 0.22; range: 0.34–2.06), with significant difference between values of PTMCs and MNGs (P
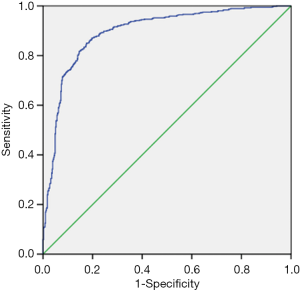
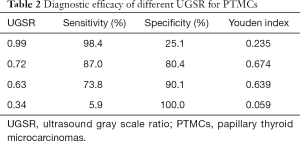
The ROC curve for the UGSR to differentiate between PTMCs and MNGs is shown in Figure 5. The AUC for the UGSR to differentiate between PTMCs and MNGs was 0.895 (95% confidence interval: 0.876–0.915). When the UGSR decreased, the sensitivity of the UGSR to diagnose PTMCs decreased, and the specificity increased (Table 2). On the contrary, when the UGSR was 1.0 the sensitivity and specificity of the UGSRs to diagnose MNGs were 25.1% and 98.6%, respectively.
Full table
Discussion
PTMCs are the most common malignant thyroid tumors that occur in people over the age of 45 (7). Some authors (8,9) suggested follow up closely for low-risk PTMCs. Ultrasound is the most important imaging method in diagnosis and monitoring of thyroid nodules (10-14). Judging the characterization of echo is more vulnerable to be affected by operator’s subjectivity compared with ultrasound features such as of morphology, microcalcification and anteroposterior/transverse diameter ratio. Low or extremely low echo is most sensitive for the diagnosis of PTMCs which may be associated with the low degree of differentiation in cancer cells, fewer interstitial components and good sound transmission in the tumor (15). However, regarding the diagnostic efficacy of a low echo on malignant nodules, reports vary widely between studies. Sharma et al. (16) reported that the sensitivity and specificity of the low echo for diagnosing malignant nodules were 93.8% and 21.8%, respectively. Ren et al. (17) found that the sensitivity and specificity of the low echo for diagnosing malignant nodules ranged from 91.7% to 95.3% and from 19.4% to 67.7%, respectively. In Kim et al.’s study (3), the sensitivity and specificity ranged from 62% to 65.7% and from 49.7% to 55.5%, respectively. In Cappelli et al.’s study (12), the sensitivity and specificity were 79% and 61%, respectively. In Moon et al.’s study (6), the sensitivity and specificity were 87.2% and 58.5%, respectively. Therefore, the specificity of the low echo in the diagnosis of malignant nodules may have limitations.
In view of the low specificity of the low echo in the diagnosis of malignant nodules, Hong et al. (18), Moon et al. (6) and Cappelli et al. (12) studied extremely low echo results. Their results indicated that, compared with the low echo, the specificity of an extremely low echo in the diagnosis of malignant nodules was improved (ranging from 90.9% to 98.8%); however, the sensitivity was low (ranging from 17.1% to 41.4%). In determining extremely low echo levels, in addition to the issue of deviation among different observers (also affecting the determination of the low echo grade), the distance between nodule and muscle can increase assessment error. If the echo intensity of nodules can be determined using values, similar to the use of CT values to reflect nodule density, the subjective assessment deviation among different observers can be then largely avoided.
Although the ultrasonic echo intensity is completely different from the ultrasound gray scale, there is a clearly positive correlation between them. With the echo intensity ranging from weak to strong, the sonogram presents the image from black to white and the gray scale value from low to high. Therefore, the echo intensity of lesions can be reflected through measuring the gray scale value on the sonogram. In 2017, Bartolotta et al. (19) analyzed benign and malignant breast lesions using S-DetectTM, which was integrated into Samsung ultrasound machine and can assess benign and malignant breast masses according to the proposed US BI-RADS features. In these features, average gray changes or histogram changes between tissue/mass area were compared by computer-aided decision-making support system, no detailed gray scale information was demonstrated and its diagnostic efficacy was assessed. So, our method was different from Bartolotta. In this study, using the gray scale ratio of thyroid nodules to surrounding normal thyroid tissue as the UGSR, the results showed that there was a statistical difference in the UGSRs between PTMCs and MNGs. In addition, the ROC curve for PTMCs and MNGs was drawn. The AUC was 0.895, and the UGSR range for PTMCs and MNGs was 0.24–1.26 and 0.34–2.06. When the UGSR decreased, the sensitivity of the UGSR for diagnosing a PTMC decreased, and the specificity increased. The best UGSR of this study was 0.72 by using AUC, the Youden index reached its maximum, the sensitivity and specificity of diagnosing PTMCs were 87% and 80.4% respectively. Although the sensitivity seemed good, but the specificity was still insufficient. For higher specificity, we selected another two-threshold value, 0.63 and 0.34 respectively, the sensitivity and specificity of diagnosing PTMCs were 73.8% and 90.1%, 5.9% and 100.0%, respectively. In addition, to compare with low echo of traditional five-grade classification, we selected the biggest UGSR of low echo, 0.99, and the sensitivity and specificity of diagnosing PTMCs were 98.4% and 25.1% respectively. Obviously, when using 0.34 as the threshold, the specificity was 100.0%, but the sensitivity was too low which was meaningless for the clinician. Using 0.99 as the diagnostic threshold could obtain a high sensitivity; however, the specificity was clearly low (16,17); when using 0.72 or 0.63 as a diagnostic threshold, diagnostic sensitivity and specificity of PTMCs were higher than previous reports (3,5,6,16-18).
In addition, when the UGSR increased, the sensitivity of the UGSR in diagnosing MNGs decreased, but the specificity increased. When UGSR was 1.0, equal to isoechoic of traditional five-grade classification, the sensitivity and specificity were 25.1% and 98.6%, respectively. The high specificity indicates that, for MNGs, the surgical procedure needs to be more carefully performed to avoid unnecessary trauma. In comparison to the description of thyroid nodules using no echo, extremely low echo, low echo, equal echo and high echo, the description using UGSR is simpler and more accurate. Moreover, during follow-up, variations in nodule echo grades can be better quantified. When combined with other sonographic features, the nature of nodules can be better assessed.
This study has some limitations. Firstly, for nodules with heterogeneous echo, or normal thyroid tissue with heterogeneous echo due to technical factors, the selection and measurement of ROI had uncertainties. In this study, the selection and measurement of the ROI was performed by two senior imaging specialists together, which is likely to have reduced this deviation. Secondly, different observers may adopt different measurement techniques, which can result in deviation. However, the images used in this study are in accordance with quality specifications, so the deviation was small. At present, the five-grade classification on nodule echo intensity is also based on the different measurement techniques. Thirdly, multiple important ultrasonic features, such as gray scale, morphology, microcalcification and anteroposterior/transverse diameter ratio and so on, are required to determine the properties of thyroid nodules, but currently, we only focused on the preliminary study of UGSR. The combination of UGSR with other ultrasonic features in differential diagnosis of thyroid nodules will be studied in our future research. Fourth, the results of this study were mainly derived from three Esaote ultrasound systems, whether or not they are applicable to different ultrasound systems from other companies need further validation study. Finally, in comparison of naked eyes judgement from other authors, we suggest that the diagnostic efficacy of UGSR was significantly higher (3,5,6,16-18), because naked eyes based on experience, our study used UGSR as quantitative data would be more reproducible.
In conclusion, this study shows that UGSR allows potential differentiation of PTMCs and MNGs through quantitative measurement. Further studies to validate these results are being pursued by the authors.
Acknowledgements
Funding: This work was supported by Hangzhou Special Science and Technology Innovation Project (20131813A08), Zhejiang Provincial Public Technology Research Project (2017C33180), Hangzhou Social Development Project of Science and Technology Commission (20180533B39).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. Written informed consent was waived due to the non-interventional and retrospective nature of this study.
References
- Park JY, Lee HJ, Jang HW, Kim HK, Yi JH, Lee W, Kim SH. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid 2009;19:1257-64. [Crossref] [PubMed]
- Cheng SP, Lee JJ, Lin JL, Chuang SM, Chien MN, Liu CL. Characterization of thyroid nodules using the proposed thyroid imaging reporting and data system (TI-RADS). Head Neck 2013;35:541-7. [Crossref] [PubMed]
- Kim GR, Kim MH, Moon HJ, Chung WY, Kwak JY, Kim EK. Sonographic characteristics suggesting papillary thyroid carcinoma according to nodule size. Ann Surg Oncol 2013;20:906-13. [Crossref] [PubMed]
- Erol B, Kara T, Gürses C, Karakoyun R, Köroğlu M, Süren D, Bülbüller N. Gray scale histogram analysis of solid breast lesions with ultrasonography: can lesion echogenicity ratio be used to differentiate the malignancy? Clin Imaging 2013;37:871-5. [Crossref] [PubMed]
- Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the Degree of Conformity of Papillary Carcinoma and Follicular Carcinoma to the Reported Ultrasonographic Findings of Malignant Thyroid Tumor. Korean J Radiol 2007;8:192-7. [Crossref] [PubMed]
- Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH. Thyroid Study Group. Korean Society of Neuro- and Head and Neck Radiology. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology 2008;247:762-70. [Crossref] [PubMed]
- Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 2011;21:231-6. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Oda H. Insights into the Management of Papillary Microcarcinoma of the Thyroid. Thyroid 2018;28:23-31. [Crossref] [PubMed]
- Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, Masuoka H, Yabuta T, Fukushima M, Higashiyama T, Kihara M, Kobayashi K, Miya A. Incidences of Unfavorable Events in the Management of Low-Risk Papillary Microcarcinoma of the Thyroid by Active Surveillance Versus Immediate Surgery. Thyroid 2016;26:150-5. [Crossref] [PubMed]
- Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892-9. [Crossref] [PubMed]
- Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, Dominguez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab 2009;94:1748-51. [Crossref] [PubMed]
- Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, Agosti B, Rosei EA. Thyroid nodule shape suggests malignancy. Eur J Endocrinol 2006;155:27-31. [Crossref] [PubMed]
- Choi YJ, Kim SM, Choi SI. Diagnostic accuracy of ultrasound features in thyroid microcarcinomas. Endocr J 2008;55:931-8. [Crossref] [PubMed]
- Brown RE, Harave S. Diagnostic imaging of benign and malignant neck masses in children-a pictorial review. Quant Imaging Med Surg. 2016;6:591-604. [Crossref] [PubMed]
- Zhang XL, Qian LX. Ultrasonic features of papillary thyroid microcarcinoma and non-microcarcinoma. Exp Ther Med 2014;8:1335-9. [Crossref] [PubMed]
- Sharma A, Gabriel H, Nemcek AA, Nayar R, Du H, Nikolaidis P. Subcentimeter thyroid nodules: utility of sonographic characterization and ultrasound-guided needle biopsy. AJR Am J Roentgenol 2011;197. [Crossref] [PubMed]
- Ren J, Liu B, Zhang LL, Li HY, Zhang F, Li S, Zhao LR. A taller-than-wide shape is a good predictor of papillary thyroid carcinoma in small solid nodules. J Ultrasound Med 2015;34:19-26. [Crossref] [PubMed]
- Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS. Positive predictive values of sonographic features of solid thyroid nodule. Clin Imaging 2010;34:127-33. [Crossref] [PubMed]
- Bartolotta TV, Orlando A, Cantisani V, Matranga D, Ienzi R, Cirino A, Amato F, Di Vittorio ML, Midiri M, Lagalla R. Focal breast lesion characterization according to the BI-RADS US lexicon: role of a computer-aided decision-making support. Radiol Med 2018;123:498-506. [Crossref] [PubMed]



