Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT
Introduction
Lung nodules are often discovered accidentally when chest imaging is performed during routine screening or for some other reason. The guidelines for management of lung nodules have been reported elsewhere (1). For malignant cancer patients with pulmonary nodules, CT follow-up is necessary after initial treatment, in order to enable timely diagnosis and effective management of lung nodules. Conventional visual methods fail to differentiate malignant from benign nodules in many cases (2). For example, if an error of observation or measurement of the longest diameter is made by the radiologist, an inappropriate classification into “no further follow-up indicated” would be rendered. Ultimately this could result in a failure to diagnose a growing lung cancer (3). So in addition to subjective observation, a computer-aided diagnosis (CAD) system may play a crucial role in the evaluation of pulmonary nodules (4).
However, the exposure to ionizing radiation from multiple CT scans can induce the risk of secondary cancer induction (5). International Commission on Radiological Protection (ICRP) suggests that every 1 mSv increase of radiation can induce the lifetime risk of cancer by 0.005% (5). Therefore, it is desirable to establish a CT scanning protocol with low-dose radiation for the surveillance of lung nodules. The guidelines published in 2013 recommended a consistent low-dose CT scan for patients who required prolonged follow-up, especially in younger patients (6). The concept of low-dose CT scan in lung was initially proposed by Naidich and colleagues. As a consequence, they found that the mortality rate of lung cancer was 20% lower in low-dose radiation group as compared to standard dose group (7).
Low-dose CT scan is typically achieved by fixing the tube voltage, lowering the tube current or reducing scan length (8-10). In addition, the automatic tube current modulation is gaining much attention and has been reported to be an effective auto-exposure control system by minimizing the radiation exposure to patient while maintaining adequate image quality (9,11). It is well known that the tube voltage has an exponential relationship with radiation dose, and thus, lowering the tube voltage can result in a significant decrease in radiation dose (12,13). However, another study focusing on the low dose and dual-energy CT has revealed that lowering the scanning voltage may cause an apparent increase in CT value of certain brain tissues (14). Other studies (15,16) have reported that the average enhanced CT values of pulmonary trunk, segment and sub-segment blood vessels were significantly higher in the low tube voltage group (110 or 100 kV) than those in the standard voltage group (130 or 120 kV).
In a previous study, lowing the tube voltage to 80 kV can obtain a high quality and evaluable CT images, while allowing a significant reduction of radiation dose by 32% to 60% (17). But it is still uncertain whether the lung nodule images scanned with low tube voltage are of comparable diagnostic accuracy as those scanned with standard tube voltage. In this study, we investigated the imaging characteristics of lung nodules with a range of 4 to 30 mm in diameter among cancer patients receiving low (80 kV) and standard voltage (120 kV) CT scans. Accordingly, this study aimed to retrospectively assess whether low-voltage lung CT scan coupled with iterative reconstruction algorithms can be an optimal scanning method for measuring the size and density of lung nodules in cancer patients.
Methods
Participants
We conducted a retrospective review of all CT images obtained from 82 (43 males, 39 females; average age: 56.16±1.16 years old; BMI: 24.55±0.34 kg/m2) cancer patients who were admitted to the Department of Medical Oncology at the First Hospital of China Medical University from March to November 2014. These patients received both low-dose lung (80 kV) and standard-dose abdominal (120 kV) CT scans for intra- or extra-lung cancer. In addition, the ROI was retrieved by the same scanning methods. The study protocol was approved by the Institutional Review Board (IRB) of the same university and local ethics committee. A waiver of informed consent was requested and approved by the IRB.
CT data acquisition
All CT scans were performed with a 256-section iCT machine (Philips Healthcare, Cleveland, OH). The scan parameters were as follows: (I) a low tube voltage of 80 kV was used for the complete spiral chest CT scanning of the lungs from apex to base; (II) a standard tube voltage of 120 kV was used for the abdominal scans and the scanning range was from diaphragmatic dome to pubic symphysis. Unless noted elsewhere, the following scan parameters were used throughout the study: rotation speed of 0.5 s/r; slice thickness of 1 mm, with 1 mm increment; a hybrid iterative reconstruction iDose4 algorithm with strength level of 2 (Philips Healthcare, Cleveland, OH) and implementation of soft tissue and lung reconstruction algorithms. The lung nodules that located in both lung and abdominal CT scans were selected for this retrospective analysis.
Image quality assessment
Image visual evaluation
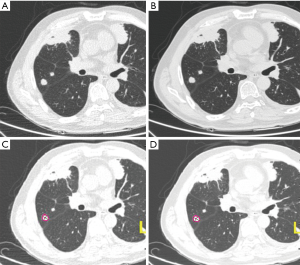
All images with lung window settings [WW, 1,500 Hounsfield units (HU); WL, −600 HU] were reviewed by two senior radiologists in a double-blind mode. The adjustments of window width and window level were allowed during image quality evaluation. The subjective 4-point scale was adopted (18), with a slight modification (Figure 1): 0= fuzzy (poor) image with strong noise and substantial artifacts, which is not useful for diagnosis; 1= fair image with partially obscured contour, moderate noise and artifacts, but with acceptable diagnostic value; 2= good image with little noise or artifacts and clear contours providing sufficient diagnostic information; and 3= excellent image with minimal noise and artifacts, and smooth contours, which is extremely useful for diagnostic purposes.
Lung nodules measurement and inclusion criteria
Single or multiple lung nodules showing clear boundaries and largest cross-sectional diameter ≤30 mm were included. Additionally, the lung nodules were surrounded by aerated lung tissue, but not associated with atelectasis, hilar enlargement or pleural effusion as described previously (12).
Images derived from standard 120 kV scan were first assessed through a computer to automatically measure the maximum diameter of lung nodules. Subsequently, all the images were transferred to the workstations (Aquarius iNtuition Edition ver. 4.4.6.80) with SAT-lung module and Lung Nodules Assessment, for the automatic measurement of lung nodule segmentation.
The inclusion criteria for lung nodule were as follows: (I) nodules measured between 4 and 30 mm; and (II) recognized by the aforementioned automatic pulmonary nodule segmentation systems. Lung nodules that met the pre-established criteria were further analyzed with a semi-automated approach for their average diameter, volume and CT value. In addition, the same images were examined by different computer-aided diagnostic (CAD) systems.
Classification of lung nodules
The lung nodules were classified according to:
- Size: lung nodules detected by both 80 and 120 kV CT scans were classified into three groups based on their maximal diameter. For 120 kV, the nodules measured between 4 and 8 mm in diameter were classified as A120kV-1; the nodules measured between 8 and 15 mm as A120kV-2; while the nodules measured between 15 and 30 mm as A120kV-3. Similarly, the nodules detected with 80 kV were classified as A80kV-1, A80kV-2 and A80kV-3 for the nodules measured between 4 and 8, 8 and 15, and 15 and 30 mm in diameter, respectively.
- Density: lung nodules identified with 80 kV and 120 kV were visually assessed by two radiologists and divided into three groups based on their density. For 120 kV, A*120kV-1: partial-solid nodules; A*120kV-2: solid nodules and A*120kV-3: calcified nodules. Likewise, for 80 kV, all the three nodule groups were denoted as A*80kV-1, A*80kV-3 and A*80kV-3, respectively. In this study, the partial-solid nodules involved the pure ground-glass opacity (GGO) nodules that can be reconstructed with 1 mm of thickness and mixed GGO nodules (i.e., partially solid nodule). Calcified nodules were consisted of calcified components with a diffused or centralized, thin or popcorn-like calcification form within the lung CT scan (19). Solid nodules appeared as an attenuating mass between partial solid and calcified nodules.
The diameter, volume and CT value of each lung nodule were obtained from the average measurement of the two CAD systems (CADs), except for the agreement between them. In order to validate the accuracy and reliability of CADs measurements, the maximum diameter and CT value of large nodules were measured independently, and the results were compared to those measured with CADs under the two different voltages.
Statistical analysis
Cohen’s Kappa statistic was used to evaluate the inter-rater reliability between the scores of two radiologists regarding the image quality obtained from both low and standard voltage protocols. Kappa value (K) was considered highly reliable if K>0.80, fairly reliable (0.61–0.80), moderately (0.41–0.60), poorly reliable (0.21–0.40) and unreliable (<0.20).
Meanwhile, the agreement between the CADs measurements was determined by intraclass correlation coefficient (ICC) and Bland-Altman plot. Paired sample t-test was used to evaluate if there was a significant difference in the CT value between low and standard voltage groups. The maximum diameter, maximum volume and the difference between CT value and maximum diameter were measured manually. All statistical analyses were performed with SPSS statistical software (SPSS Version 16.0, Chicago, IL). Data were expressed as mean ± SEM. P values of less than 0.05 were considered statistically significant. The actual value for the percentage difference can be calculated from the formula below:
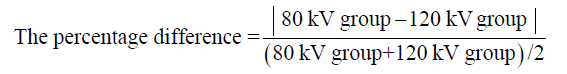
Results
Image quality assessment
Images acquired with 80 and 120 kV scan protocols
CT scan images acquired from 80 and 120 kV exhibited a clean margin that facilitates an accurate detection of lung nodule. Moreover, no obvious differences were observed for the images obtained from the two different voltage protocols following algorithm analysis. However, a few beam hardening artifacts were found in some images acquired with 80 kV (Figure 2).
Subjective image assessment
The images obtained from 82 patients were assessed and scored by two radiologists. The results are summarized in Table 1. All images received 2 points or higher, except for two cases who received 1 point for their relative poor image quality, indicating that the scanned images are of high quality and optimal diagnostic values. Moreover, a fairly reliable agreement was reached between two inter-observers, as demonstrated by strong K values of 0.848 and 0.829. The mean score of the images obtained with 120 kV was 2.51±0.053; while 2.35±0.054 for 80 kV scans. However, there was no significant difference in the mean score between the two voltage protocols (P>0.05).

Full table
Detection rate of lung nodules
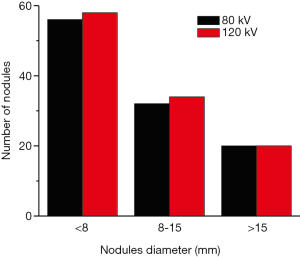
Under the tube voltage of 120 kV, a total of 112 nodules were identified from 63 of 82 patients, with a detection rate of 76.83%. When a low tube voltage of 80 kV was applied, the nodule detection rate was 74.39% (108 nodules from 61/82 patients), which was almost identical with the standard tube voltage of 120 kV. However, χ2 test revealed that the observed frequency distribution for the two voltage protocols did not differ significantly (t=0.716, P>0.05).
Measurements of lung nodule diameter with CAD systems
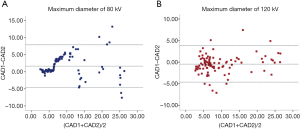
Through visual inspections, our results suggest that the detection rate of lung nodules is identical between the two voltages (Figure 3). We further evaluated the measurements of maximum diameter of 96 lung nodules (small, n=52; medium, n=26; and large, n=18 nodules) using two commercial CAD software packages (SAT-lung of Aquarius iNtuition Edition (ver. 4.4.6.80, Premier Farnell) and Lung Nodule Assessment, Philips). The mean diameters of lung nodules analyzed by SAT-lung were (120 kV: 9.71±0.654 mm and 80 kV: 9.64±0.677 mm), while the mean diameters were 9.44±0.689 mm (120 kV) and 9.51±0.647 mm (80 kV) for Lung Nodules Assessment. However, no significant differences were observed for the mean diameters of lung nodules assessed by the two CAD systems under the same voltage scan. Furthermore, the degree of agreement was determined by calculating the ICC values, in order to compare the results between SAT-lung and Lung Nodules Assessment (Figure 4). The obtained ICC values for 120 and 80 kV were 0.957 and 0.90, respectively. In addition, Bland-Altman plots suggest that only 4 out of 96 nodules (80 kV) and 6 out of 96 (120 kV) lie beyond the 95% confidence intervals (Figure 5), indicating that a fairly good agreement is existed between the two CAD systems, for the measurement of nodule diameters.
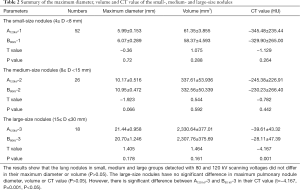
Comparison of maximum diameter, volume and CT value in lung nodules with different size
As shown in Table 2, a total of 96 lung nodules were analyzed for their maximum diameter, volume and CT value at different voltage scan. These results indicated that there were no significant differences in the maximum diameter and volume of lung nodules among the small-, medium- and large- size groups (P>0.05). Interestingly, a significant difference (P<0.05) was observed for the CT value of lung nodules in large size group, but not small and medium size groups (P>0.05). On average, CT value at 80 kV was 33.96% higher than that at 120 kV.
Full table
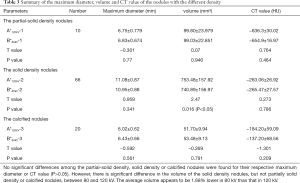
Comparison of maximum diameter, volume and CT value in lung nodules with different density
The maximum diameter, volume and CT value of lung nodules with different density at different voltage scan are listed in Table 3. No significant differences were found among the partial-solid density, solid density and calcified nodules groups with regards to their maximum diameter and CT value (P>0.05). There was a significant difference in the volume of solid density nodules between 80 and 120 kV (740.89±156.97 vs. 753.48±157.92 mm3, respectively; P<0.05), but not partially solid density and calcified nodules. The average volume at 80 kV appeared to be 1.68% lower than that at 120 kV.
Full table
Validation of diameter measurements of the large nodules by CADs
The diameters and CT values of 18 nodules from large-size group were measured and compared to estimate the degree of agreement between manual measurement and automatic CADs measurement. The results are shown in Table 4, indicating that the accuracy and reliability of measurements for lung nodule diameter can be achieved with automated CAD systems. Notably, the performance of manual measurement is considerably lower than of CAD systems.
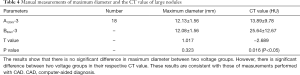
Full table
Discussion
To date, with the increasing frequency of CT examination in routine clinical practice and in accordance to the as-low-as-reasonably-achievable principle, much progress has been made in the reduction of radiation dose (7,9,20,21). Moreover, in patients with primary extra pulmonary tumors, long-term CT-scan follow-up are often required to detect and monitor metastatic lung cancer. Except for those lung nodules measuring greater than 30 mm in diameter, the nodules with diameters between 4 and 30 mm should be monitored by multiple CT scans (22,23). However, CT follow-up is not necessary for low risk cancer patients with nodule diameter of less than 4 mm, as recommended by Fleischner Society (6,24,25). Hence, our study focused on the intra- or extra-pulmonary lung cancer patients with nodules of less than 30 mm in diameter. By taking advantage of the fact that the lung nodules have been scanned at both low and standard voltages, we retrospectively assessed the scan images to determine whether the images acquired with low voltage scan can demonstrate an adequate quality for clinical diagnostic purposes.
Our findings strongly support the low dose 80 kV CT scan as an essential diagnostic imaging tool for a continually increasing variety of clinical applications. Additionally, the feasibility and safety of such low voltage protocol are subjected to a continuous surveillance of patients with lung nodules. The results of indicated a reliable inter-observer agreement for the quality of images scored at different voltages scan. Moreover, CT images acquired at low voltage were sufficient to assess the lung nodules without compromise diagnostic confidence. Furthermore, the lung nodule images with low voltage scan can exhibit an identical detection rate as compared to those with standard voltage scan, suggesting that 80 kV scan protocol can be used for multiple CT scans as long as the size of the nodule is concerned. These results were consistent with previous findings of low-dose CT scan. For instance, Adrian et al. evaluated an ultra-low dose CT scan for lung disorders, and their results showed that the low-dose of 80 kV CT is clinically useful for lung cancer screening as compared to the conventional dose of 100 kV (26).
In theory, the diameter of nodule may increase by 26% when the volume is doubled. Thus, the volume measurement appears to be more sensitive than the changes in nodule’s diameter, especially for small nodules. Our results showed that there was no difference in the diameter of nodules scanned at low and high tube voltage. However, the volume of the solid nodules was significantly different between the two voltages group. The volume of lung nodules measured in 80 kV is smaller than that in 120 kV group, with a slight difference of 1.68%. These results, however, were contrary to what we anticipated. Such discrepancy may be attributed to the asymmetrical growth of pulmonary nodules (27,28) and the altered density by different tissue textures surrounding lung nodules. For example, the calcified nodules appear to be larger in high tube voltage than in low tube voltage. Furthermore, the volume measurement of lung nodules was carried out by using semi-automated CAD systems, in order to eliminate inter-observer variability (23,29). Indeed, the two types of 3D volume measurement methods with different algorithms are able to enhance the diameter measurement of lung nodules, which, in accordance with the results from other studies (30,31).
It is important to note that slight more streak artifacts appear to be visible in the images acquired with 80 kV than those with 120 kV. Notably, CT value tends to be a little higher in the images acquired at 80 kV than 120 kV. Some of the CT values were negative, particularly in small nodule groups. Typically, the entire area of a small nodule was chosen for measurements, instead of targeting the region of interest (ROI) of a well-defined nodule. Such approach can be problematic for analyzing small nodules, especially when a manual measurement is attempted. Although the average CT value of lung nodules with low density background can be attenuated by CAD algorithm, the objectivity of measurements may be adequately maintained with CAD due to the preservation of selected axial section for maximum area. On the other hand, the results obtained from the large nodules further confirmed that the measurements performed either manually or by CAD are in a good agreement, implicating the accuracy and reliability of CAD measurement. Besides, the noises generated from low voltage scan can be improved with iterative reconstruction algorithms (20,26). Furthermore, the mean difference of CT value was 33.96% for CAD measurement and 59.82% for manual measurement. Indeed, lowering the tube voltage may result in the increase of CT value for nodules with diameter of >15 mm, due to the size of nodules, partial volume effects and edge effects (32).
In the present study, the nodule surrounded by air-filled tissues can interfere with the accuracy of measurements, which lead to the deviation of CT value. Thus, these findings should be interpreted with caution prior to clinical application. Another limitation of the current study is that the latest commercial model based IR algorithm (IMR) was not implemented for imaging analysis, due to its unavailability. IMR is considered to be superior than both Filtered Back Projection (FBP) and iDose4 in terms of image noise reduction, contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR) at different radiation dose settings (32). Hence, it is anticipated that IMR technique can minimize radiation dose while maintaining diagnostic image quality.
Conclusions
In conclusion, our findings strongly suggest that the CT scan with low voltage allows a safe and accurate assessment of lung nodules in cancer patients. Low voltage CT scan coupled with the powerful iterative reconstruction algorithm analysis represents a novel method for evaluation of lung nodules with different size or density. Additionally, this newly developed method is suitable for patient who requires prolonged follow-up on chest CT. Automated Image analysis of lung nodules using two commercial software systems yielded substantially the same results as those obtained manually. However, the differences in the CT value of large nodules and the volume of solid nodules should be interpreted with caution due to the relatively small sample size. Therefore, future investigation with a larger sample size is warranted to determine the possible causes of the unanticipated or unexpected findings.
Acknowledgements
We would like to express heartfelt thanks to all members of our research group.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board (IRB) of the same university and local ethics committee A waiver of informed consent was requested and approved by the IRB.
References
- MacMahon H, Naidich DP, Goo JM, Lee KS, Leung AL, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, Rubin GD, Schaefer-Prokop CM, Travis WD, VanSchil PE, Bankier AA. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Dennie C, Thornhill R, Sethi-Virmani V, Souza CA, Bayanati H, Gupta A, Maziak D. Role of quantitative computed tomography texture analysis in the differentiation of primary lung cancer and granulomatous nodules. Quant Imaging Med Surg 2016;6:6-15. [PubMed]
- Gavrielides MA, Berman BP, Supanich M, Schultz K, Li Q, Petrick N, Zeng R, Siegelman J. Quantitative assessment of nonsolid pulmonary nodule volume with computed tomography in a phantom study. Quant Imaging Med Surg 2017;7:623-35. [Crossref] [PubMed]
- Cavalcanti PG, Shirani S, Scharcanski J, Fong C, Meng J, Castelli J, Koff D. Lung nodule segmentation in chest computed tomography using a novel background estimation method. Quant Imaging Med Surg 2016;6:16-24. [PubMed]
- Boyd MA. US radiation protection: role of national and international recommendations and opportunities for collaboration (harmony, not dissonance). Health Phys 2015;108:278-82. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo J M, Macchiarini P, Crapo JD, Herold CJ, Austin JH, Travis WD. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Naidich DP, Marshall CH, Gribbin C, Arams RS, McCauley DI. Low-dose CT of the lungs: preliminary observations. Radiology 1990;175:729-31. [Crossref] [PubMed]
- Campbell J, Kalra MK, Rizzo S, Maher MM, Shepard JA. Scanning beyond anatomic limits of the thorax in chest CT: findings, radiation dose, and automatic tube current modulation. AJR Am J Roentgenol 2005;185:1525-30. [Crossref] [PubMed]
- Kalra MK, Maher MM, Toth TL, Schmidt B, Westerman BL, Morgan HT, Saini S. Techniques and applications of automatic tube current modulation for CT. Radiology 2004;233:649-57. [Crossref] [PubMed]
- Nitta N, Takahashi M, Murata K, Morita R. Ultra low-dose helical CT of the chest: evaluation in clinical cases. Radiat Med 1999;17:1-7. [PubMed]
- Kalra MK, Naz N, Rizzo SM, Blake MA. Computed tomography radiation dose optimization: scanning protocols and clinical applications of automatic exposure control. Curr Probl Diagn Radiol 2005;34:171-81. [Crossref] [PubMed]
- Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S, Kastrati A, Schomig A. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation 2006;113:1305-10. [Crossref] [PubMed]
- Kalender WA, Buchenau S, Deak P, Kellermeier M, Langner O, van Straten M, Vollmar S, Wilharm S. Technical approaches to the optimisation of CT. Phys Med 2008;24:71-9. [Crossref] [PubMed]
- Peng W, Peng T, Xia S, Wang X, Jia Q, Fu Y, Mu G. The effects of X-ray tube voltage on tissue CT value. Radiologic Practice 2013;28:1102-4.
- Heyer CM, Mohr PS, Lemburg SP, Peters SA, Nicolas V. Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 2007;245:577-83. [Crossref] [PubMed]
- Matsuoka S, Hunsaker AR, Gill RR, Oliva IB, Trotman-Dickenson B, Jacobson FL, Hatabu H. Vascular enhancement and image quality of MDCT pulmonary angiography in 400 cases: comparison of standard and low kilovoltage settings. AJR Am J Roentgenol 2009;192:1651-6. [Crossref] [PubMed]
- Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF. Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 2004;231:169-74. [Crossref] [PubMed]
- Ren F, Fu K. Application of Low-dose Chest Spiral CT in Screening Pulmonary Nodules in Healthy Population. Chin Comput Med Imag 2014;20:389-93.
- Li M, Jirapatnakul A, Biancardi A, Riccio ML, Weiss RS, Reeves AP. Growth pattern analysis of murine lung neoplasms by advanced semi-automated quantification of micro-CT images. PLoS One 2013;8. [Crossref] [PubMed]
- Funama Y, Taguchi K, Utsunomiya D, Oda S, Yanaga Y, Yamashita Y, Awai K. Combination of a low-tube-voltage technique with hybrid iterative reconstruction (iDose) algorithm at coronary computed tomographic angiography. J Comput Assist Tomogr 2011;35:480-5. [Crossref] [PubMed]
- Khawaja RD, Singh S, Madan R, Sharma A, Padole A, Pourjabbar S, Digumarthy S, Shepard JA, Kalra MK. Ultra low-dose chest CT using filtered back projection: Comparison of 80-, 100- and 120 kVp protocols in a prospective randomized study. Eur J Radiol 2014;83:1934-44. [Crossref] [PubMed]
- Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014;106. [Crossref] [PubMed]
- Marten K, Engelke C, Seyfarth T, Grillhosl A, Obenauer S, Rummeny EJ. Computer-aided detection of pulmonary nodules: influence of nodule characteristics on detection performance. Clin Radiol 2005;60:196-206. [Crossref] [PubMed]
- Baldwin DR, Callister ME. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF, Swensen SJ. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [Crossref] [PubMed]
- Huber A, Landau J, Ebner L, Bütikofer Y, Leidolt L, Brela B, May M, Heverhagen J, Christe A. Performance of ultralow-dose CT with iterative reconstruction in lung cancer screening: limiting radiation exposure to the equivalent of conventional chest X-ray imaging. Eur Radiol 2016;26:3643-52. [Crossref] [PubMed]
- Yankelevitz DF, Gupta R, Zhao B, Henschke CI. Small pulmonary nodules: evaluation with repeat CT-preliminary experience. Radiology 1999;212:561-6. [Crossref] [PubMed]
- Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217:251-6. [Crossref] [PubMed]
- Jeon KN, Goo JM, Lee CH, Lee Y, Choo JY, Lee NK, Shim MS, Lee IS, Kim KG, Gierada DS, Bae KT. Computer-aided nodule detection and volumetry to reduce variability between radiologists in the interpretation of lung nodules at low-dose screening computed tomography. Invest Radiol 2012;47:457-61. [Crossref] [PubMed]
- de Hoop B, Gietema H, van Ginneken B, Zanen P, Groenewegen G, Prokop M. A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: what is the minimum increase in size to detect growth in repeated CT examinations. Eur Radiol 2009;19:800-8. [Crossref] [PubMed]
- Goo JM. A computer-aided diagnosis for evaluating lung nodules on chest CT: the current status and perspective. Korean J Radiol 2011;12:145-55. [Crossref] [PubMed]
- Weng CZ. Reasons for artifacts in CT and its solution. Chinese Medical Equipment Journal 2006;27:49-51.


