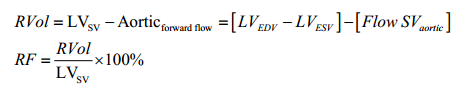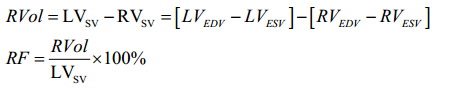Multimodality imaging for the quantitative assessment of mitral regurgitation
Introduction
Mitral regurgitation (MR) is the second most frequent valve disease in Europe after aortic valve stenosis (1,2). Although some patients may remain asymptomatic, severe MR eventually leads to left ventricular (LV) failure, pulmonary hypertension, atrial fibrillation and death (3). The degree of MR is defined by the lesion severity [measured as effective regurgitant orifice area (EROA)] and the resulting volume overload [measured as regurgitant volume (RVol)] (4). Patients referred to surgical centres for severe MR, based on echocardiography findings, are often found to have only mild or moderate MR on quantitative evaluation (5). Accurate assessment of MR severity and its complications are important, as it not only determines timing and indication for surgical correction, but also carries significant prognostic implications (3,6).
Traditionally, imaging has focused on assessing mitral valve (MV) morphology, hemodynamic severity, ventricular remodelling and suitability for surgical intervention. Recent innovations in non-invasive imaging have provided insights into the quantification of MR, early detection of LV dysfunction, and advanced prognostic assessment; these are potentially additional factors for determining surgical timing in asymptomatic MR. This review examines the role and limitations of contemporary non-invasive imaging modalities for the assessment of patients with MR.
Imaging modalities
The comprehensive assessment of MR requires evaluation of MV anatomy, MR severity, LV size and systolic function, and assessment of associated features such as pulmonary arterial hypertension. Echocardiography, which includes both transthoracic (TTE) and transesophageal (TOE) approaches, has been the cornerstone of assessing MR, providing anatomical and functional information. In most instances, the use of 2D and Doppler echocardiographic protocols are sufficient. However, echocardiographic methods have their limitations as they are based on many geometric assumptions, resulting in less accurate quantification of LV function and MR severity. Advanced cross-sectional imaging modalities such as cardiovascular magnetic resonance (CMR) imaging and multi-slice computed tomography (CT) are increasingly useful when echocardiographic imaging is suboptimal and may provide supplementary information in selected patients.
Echocardiography
Echocardiographic assessment of MR can determine its aetiology and mechanism, assess its severity as well as the hemodynamic consequences on the left ventricle (4). An integrated and comprehensive assessment of MR requires the following evaluation: (I) MV anatomy; (II) qualitative findings for MR severity; (III) quantitative findings regarding RVol and EROA; (IV) LV size and function; (V) other supportive findings that may determine prognosis or feasibility of successful surgical repair, for example, sub-valvular apparatus or extent of calcification (4), right ventricular function, pulmonary arterial pressure (PAP) and intra-cardiac flows.
Transthoracic echocardiography
Assessment of MR severity
Accurate grading of MR severity is essential, as current guidelines only recommend surgical referral when MR is severe by standardised criteria (7,8). Both American Society of Echocardiography (ASE) and European Society of Cardiology (ESC) guidelines recommend integrating multiple qualitative, semi-quantitative and quantitative echocardiographic parameters when assessing MR severity; although each has their inherent limitations (9,10). Criteria for descriptive and semi-quantitative grading are shown in Figure 1.
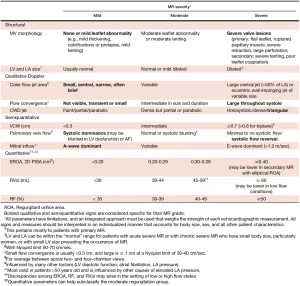
Qualitative assessment
Color flow Doppler
Although mild MR [a small jet confined to early or late systole with small/absent flow convergence and a narrow vena contracta (VC)] can easily be diagnosed with color flow imaging, qualitative assessment of larger or more eccentric jets is challenging. Atrial size is inherently linked to atrial pressure and compliance, both of which may themselves affect jet area (11). Eccentric jets commonly project against the atrial wall, exhibit a thin dimension perpendicular to the wall (Coanda effect) and therefore cannot be reliably assessed (11) (Figure 2). This technique should therefore not be used for grading MR severity. If more than a small central jet is observed, measurement of the VC and the flow convergence method [proximal isovelocity surface area (PISA)] is recommended (12).
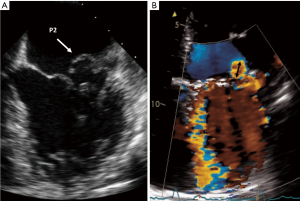
Continuous wave (CW) density jet
The CW Doppler envelope of the MR signal can provide clues to lesion severity. As the intensity of the Doppler signal is proportionate to the number of scatterers (i.e., red blood cells) in the beam, severe MR with large regurgitant volumes will generally produce high intensity Doppler envelopes (11). A dense CW-Doppler signal of the MR jet is consistent with severe MR. Nevertheless, there are several limitations to this method. Firstly, there are no specific criteria for the designation of moderate MR, other than the absence of findings consistent with either mild or severe MR (13). Secondly, interpretation of color flow patterns is subjective, thus blurring the distinction between moderate and severe (13). As signal density depends on spectral recording of the jet, a central jet well aligned with the ultrasound beam may appear denser than an eccentric jet of much greater severity (9). Thirdly, although specific signs have high positive predictive value, they lack sensitivity for the detection of severe MR (9). These limitations have led to the development of quantitative methods for assessment of MR.
Semi-quantitative assessment
VC width
The VC, defined as the narrowest portion of the MR regurgitant jet, reflects the regurgitant orifice area and therefore predicts the severity of MR (11). The relation between VC width and EROA has previously been confirmed (14,15), and appears to hold true even in eccentric MR (16). A VC width <3 mm is considered as mild MR, whereas a width ≥7 mm indicates severe MR. Intermediate values require confirmation by another approach, such as the PISA method. Because of the small values of the width of the VC (usually <1 cm), small errors in its measurement may lead to a large percentage error and misclassification of the severity of regurgitation (9).
Pulmonary vein flow/mitral inflow
Pulmonary venous systolic flow reversal, a peak mitral E-velocity >1.5 m/s (in the absence of mitral stenosis) and a pulsed-wave (PW) Doppler mitral to aortic velocity time integral ratio of >1.4 are additional indicators in favor of severe MR (11).
Quantitative assessment
All international guidelines (7,9,10,17) recommend quantitative methods, which measure the RVol, regurgitant fraction (RF) and the EROA, as these appear to have greater accuracy. Quantitation is based on hydrodynamic principles which rely on the non-compressibility of blood and the conservation of mass principle. Flow can be calculated as: flow = (vessel area) × (mean velocity of blood) (13).
Geometric assumption concepts are used to measure three parameters indicative of MR severity (13):
- EROA: the mean area of the systolic regurgitant orifice, a measure of lesion severity;
- Mitral RVol: the volume regurgitated in each systole (mL/beat), a measure of absolute volume overload;
- Mitral RF: the percentage of the total LV stroke volume represented by the RVol, a measure of relative volume overload.
In order to derive the above quantitative parameters of MR severity, echocardiography uses these three validated methods:
- PW Doppler.
RVol is calculated as the difference between mitral and aortic stroke volume (18); RF is noted as the ratio of RVol to mitral stroke volume, and EROA as the ratio of RVol to the regurgitant jet velocity-time-index (13). In the calculations of stroke volume, both mitral annular area and LVOT are assumed to be circular in geometry (11). Incorrect diameter measurements will result in large errors since the value must be squared to generate the cross sectional area (11). - Volumetric method.
RVol is calculated as the difference between LV stroke volume and aortic stroke volume (9). The potential pitfall of this method is the underestimation of true LV volume (i.e., due to foreshortening or unclear endocardial borders) therefore underestimating regurgitation severity (9). The use of 3D echocardiography may improve the accuracy of LV volume determinations (9). - PISA.
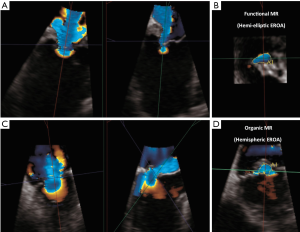
This method focuses on the flow convergence proximal to the regurgitant orifice as observed with color-flow imaging; where PISA radius of the convergence zone can be derived. Flow through the convergence zone is presumed to be equivalent to the flow through the regurgitant orifice. The use of CW-Doppler of the MR jet allows calculation of the EROA and the RVol (13,19). Despite its objectiveness, there are again some limitations associated with this method. Since the PISA calculation provides an instantaneous peak flow rate, the EROA calculated by this approach may not be equivalent to the average regurgitant orifice throughout the regurgitant phase (9). Additionally, there are assumptions that the valvular plane from which the regurgitant orifice arises is planar and that the flow convergence is homogeneous, although this is not always the case. In cases where the regurgitant orifice is noncircular, as frequently is seen in functional MR (crescent shape), the PISA shape is no longer hemispheric (9). Application of the standard PISA formula to such an elliptical orifice will lead predictably to flow underestimation (9) (Figure 3). 3D color-flow would provide a better assessment of the PISA surface, although with additional limitations of lower spatial and temporal resolution (9).
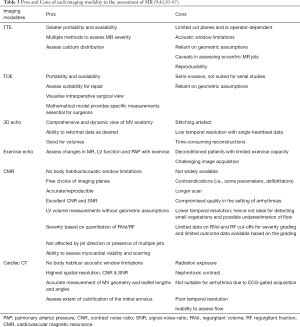
The advantages and limitations of each echocardiographic parameter used to quantify MR severity are summarised in Table 1. A detailed description of the methodology and equations for how these values are derived are beyond the scope of this review article, but can be found in the relevant literature (6,9,18,22-25).
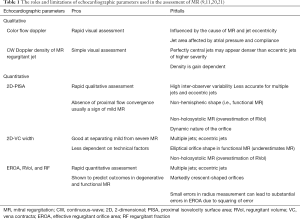
Full table
Challenges for the quantitative assessment of secondary MR
MR can be classified as primary (organic) or secondary (functional) MR. Primary MR is caused by intrinsic valve lesions (i.e., degenerative/prolapse/flail), rheumatic disease or endocarditis (26,27); whereas secondary MR results from LV remodelling, commonly seen in dilated cardiomyopathy or in ischaemic heart disease (19). Secondary MR can be much more challenging to grade than primary MR. The total LV forward stroke volume may be reduced and thus RVol is usually lower than in primary MR (<60 mL for severe MR if total stroke volume is reduced). Although RF would account for comparative lower flows, its derivation has higher errors due to the small numbers involved (28,29). The regurgitant orifice is also frequently semilunar or elliptical, affecting measurements of VC width and possibly leading to underestimation of EROA by the 2D-PISA method. Additionally, EROA may vary with LV size and LV ejection fraction (LVEF) (30). Thus, in the setting of secondary MR, whilst EROA ≥0.4 cm2 still denotes severe MR, a lower cut-off of EROA ≥0.2 cm2 may still be likely severe MR due to the above considerations (30). Adding to the challenges, adjunctive findings are also less helpful because they are often rendered abnormal by the underlying cardiac pathology. For example, most patients with cardiomyopathy have systolic blunting of the pulmonary venous flow pattern due to elevated left atrial (LA) pressure. Another confounding problem is that secondary MR is frequently very dynamic. It is therefore important to consider volume status, blood pressure, and other clinical variables in this context (9).
LVEF and LV dimensions
LVEF remains one of the strongest prognostic factors for patients with MR, where mortality is inversely proportional to LVEF (7,17). Estimated LVEF is determined via the Simpsons bi-plane method, whereas LV dimensions are measured using the M-mode method in the parasternal long axis view. An increased LV end-systolic dimension (>40 mm) and an LVEF <60% are indicators of LV systolic dysfunction, poor prognosis, and suggest surgical correction even in the absence of symptoms (7,8). The volume to which the LV contracts at the end of systole is independent on pre-load and is determined by contractility, afterload, and eccentric remodelling. Thus, LV end-systolic dimension and volume are independent factors confounding the use of LVEF in assessing ventricular function (13).
Identifying subclinical LV dysfunction
In the context of emptying into a low impedance LA, the LVEF can remain normal for a long period of time whereas LV contractility (i.e., the innate ability of the myocardium to generate force) might already be significantly reduced (31). Some studies, including a large multicentre study, found that post-operative outcome is improved if patients are operated before LV dysfunction is established (32,33). It is therefore important to identify the early decline of LV contractility, a stage when correction of MR can be undertaken to prevent irreversible myocardial damage.
Exercise/stress echocardiography
MR is load dependent and its severity can have a dynamic nature which may increase with exercise (34). Exercise/stress echocardiography such as supine-bike exercise, can be used to examine the changes in MR severity and PAP with activity, especially in asymptomatic patients (35-37). The 2016 European Association of Cardiovascular Imaging (EACVI) and ASE guidelines recommend consideration of exercise stress echocardiography when symptoms are disproportionate to the severity of MR at rest (34). The increase in MR severity (≥1 grade), dynamic pulmonary hypertension (systolic PAP ≥60 mmHg) and limited RV contractile recruitment (TAPSE <19 mm) are all markers of poor prognosis (34,36). On the other hand, the ACC/AHA guidelines (17) recommend exercise/stress echocardiography in those with asymptomatic severe MR in order to identify high-risk individuals who may benefit from early elective surgery (class IIa level C). An increase in in EROA (≥13 mm2) or systolic PAP (≥60 mmHg) during exercise have been shown to be associated with decreased symptom-free survival (9,38).
In the setting of secondary MR, stress echocardiography may provide helpful information in the following patients: (I) dyspnea on exertion disproportionate to LV systolic dysfunction or MR severity at rest; (II) recurrent and unexplained acute pulmonary edema; (III) intermediate severity MR patients scheduled for coronary artery bypass grafting (to identify those who may benefit from combined revascularisation and MV repair); (IV) for individual risk stratification (34). Unless suspicious of ischaemic MR, there is currently no role for pharmacologic stress echocardiography (i.e., dobutamine) to evaluate severity of MR as its effects on MR severity are not considered physiological (34).
Transesophageal echocardiography
In severe asymptomatic MR, optimal outcomes are achieved in centres where MV repair rates are high (>95%) and mortality is low (<1%) (8). Assessing the feasibility of successful surgical repair is therefore crucial (3). TOE is able to provide useful information concerning the likelihood of MV repair (i.e., localisation of prolapse and chordal rupture) when TTE is of poor quality or when complex, calcified, or endocarditic lesions are suspected (3,4,39). TOE is recommended in the intra-operative setting for further diagnostic refinement (12) and is also an indispensable imaging tool for guiding percutaneous MV procedures (3). Apart from delineating anatomy of lesions and guiding deployment of the device, it also provides information on its hemodynamic dysfunction pre- and post-repair (3,13). 3D-TOE offers considerable value in localising valve prolapse/flail leaflet and in simulating a ‘surgeon’s view’ of the valve, by orientating the image to exhibit the aortic valve at the 11-o’clock position (40). It is however important to note that TOE is semi-invasive and therefore not suited for serial studies (41).
2D versus 3D echocardiography
Although 2D-echocardiography is the imaging modality of choice for the evaluation of MR severity, it can be affected by limited cut-planes and it is operator-dependent (3). Due to its foreshortened views and geometrical assumptions, 2D-echo consistently underestimates LV volumes (19). In contrast, simultaneous multi-plane imaging by 3D-echocardiography permits accurate localisation of valve lesions (3,4,12). Despite its lower spatial resolution, it is far superior in the assessment of complex MV pathology especially in the intra-operative setting (42,43). When compared with independent reference imaging modalities (i.e., radionuclide ventriculography or CMR), 3D-echo has been shown to be more accurate and reproducible than 2D-echo in the measurements of LV volumes and LVEF (19,44). PISA can also be viewed in its entirety, obviating the need to make hemispherical shape assumptions for surface area computations.
2D versus 3D parameters for MR severity
Several studies have compared the parameters used in the grading of MR severity. The largest study (n=221), using CMR as a reference standard, demonstrated that 2D-PISA method significantly under estimated RVol compared with the 3D-PISA method (55.3±19.6 versus 67.4±29.1 mL) (45). These differences were more pronounced in patients with severe MR, eccentric regurgitant jet, and asymmetrical regurgitant orifice. Matsumura et al. (n=54) (46) found that 3D-PISA more accurately quantifies EROA in MV prolapse but interestingly underestimates EROA by 24% in functional MR compared with 2D-quantitative Doppler. This underestimation can be explained by the “elongated” geometry of PISA in functional MR instead of the ‘hemispheric’ assumptions used in its calculation (47). This implies that in patients with functional MR, the calculation of EROA should be based on the 2D-quantitative Doppler instead of 3D-PISA method. In eccentric jets however, 3D-EROA planimetry was demonstrated to be superior over the 2D-PISA EROA method (48,49). Although TTE has been a mainstay of MR assessment, it has limited reproducibility (20,50) and relies on mathematical assumptions of LV geometry and cavity size, which may not apply in a remodelled ventricle. A more objective, quantification of MR severity can be obtained with CMR imaging.
CMR
CMR imaging is the reference standard non-invasive imaging modality for the assessment of ventricular volumes and ejection fractions, and has the additional capabilities of quantifying flow (allowing accurate assessment of valvular regurgitation) (51,52). Over the last decade, CMR has been shown to be a robust method of determining the severity of MR, especially in the absence of other valvular lesions (53-55). It is also able to reliably determine MR RVol irrespective of MR jet geometry and has generally a high inter-observer and inter-study reproducibility, making it ideal for serial assessment (9,56,57). In the case of ischaemic MR, CMR can assess for ischaemia, regional wall motion abnormalities and myocardial viability (58-61). Some studies also suggest that focal fibrosis may be used as an early marker of LV systolic dysfunction (62). Recent work by Myerson et al. suggests that quantitative CMR measures of RVol/RF may better predict the need for future surgery than echocardiography (63).
CMR has a number of unique advantages: it provides a view of the entire heart without limitations of body habitus or imaging windows, allows free choice of imaging planes, is free of ionizing radiation and does not require contrast administration (9). CMR should therefore be considered in patients with suboptimal echocardiographic imaging or when there is a degree of uncertainty in the severity of MR, usually in the case of eccentric jets that can be underestimated by echocardiography (41,64).
The limitations of CMR include its inability to be performed in patients with certain implanted devices (65). Since most CMR acquisitions are acquired over multiple cardiac cycles, arrhythmias such as atrial fibrillation or premature ventricular contractions may pose a challenge for standard breath-held phase-contrast velocity encoded CMR sequences (9). CMR is also not as readily available as echocardiography, cannot be performed at the bedside or in some patients with claustrophobia, and is generally a more expensive modality. One other limitation includes the inability to assess pressures inside a vessel or cardiac chamber. Although CMR can be a good alternative to CT prior to MV surgery (or transcatheter MV repair/replacement), it does not show the degree of calcification well and its spatial resolution does not permit robust assessment of coronary artery anatomy (41).
Mechanism of MR
Like echocardiography, CMR can identify morphologic abnormalities of the MV apparatus. The presence of billowing, prolapse or flail segments can be identified by dedicated cine imaging performed through the different scallops of the MV leaflets (66). In secondary MR, CMR offers accurate assessment of LV dilation and function in addition to identification of myocardial and papillary muscle scar (67). Due to lower spatial and temporal resolution, imaging of the mitral sub-valvular apparatus (i.e., flail leaflet) with CMR is suboptimal (68). It is also not ideal for detecting vegetations which can be small and highly mobile. CMR however has been shown to have good agreement with TOE with regard to valve leaflet characterization and has the ability to cross-cut the valve in any plane in order to characterize the aetiology of the MR (66,68). Although visualisation of MV structure and motion is more reliable by echocardiography, CMR is more accurate than echocardiography in quantifying the severity of MR (53,69), as recently demonstrated in a prospective multicentre trial (70).
Assessing severity of MR with CMR
MR can be assessed with CMR by qualitative, semi-quantitative or quantitative methods. As a crude guide to severity, the extent of signal loss due to spin dephasing can be visually observed in the LA on cine CMR acquisitions (71-74) (Figure 4). Alternatively, planimetry of the anatomical regurgitant orifice area (AROA) from the cine CMR acquisitions of the valve can be performed (71,75). AROA planimetry is however time consuming and remains challenging because of appropriate plane alignment and angulation. Quantitation of MR severity (i.e., RVol) is the most robust method of CMR assessment of regurgitation and can be derived using the three different CMR techniques (direct/indirect) described below (55,76,77). Direct assessment of flow in the MV (Method 1—phase contrast technique) is often less accurate due to the significant motion of the MV plane during systole (78). For this reason, quantification of RVol is more commonly performed using the indirect approach, either by comparing ventricular stroke volume to aortic forward flow (Method 2) or comparing LV and RV stroke volumes (Method 3) (9).
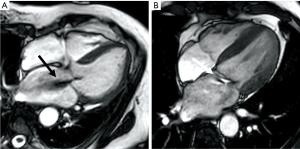
Quantitative assessment of MR severity
- Phase contrast imaging of the MV.
Phase-contrast velocity-encoded mapping is traditionally used to measure blood flow (79). A velocity image, also known as phase map, is generated in which pixel intensity depends upon the velocity of blood flow (different phase value) (79). Although this method of quantifying flow is considered the reference standard technique, it is however reliant on the ability to transect the jet at 90° in a single direction, and therefore can underestimate flow if this is not achieved (69,80). Direct measurement of the MR jet can be performed with this method by aligning the plane to the MR jet, but this can be challenging due to jet eccentricity, multiple jets and jet turbulence (81). - Difference between LV stroke volume and aortic forward flow volume.
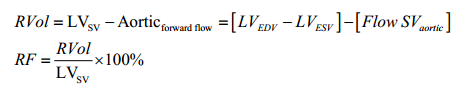
RVol is derived by calculating LV stroke volume (SV) from the short axis cine stack [end diastolic volume (EDV) − end systolic volume (ESV)] and deducting the aortic forward flow derived from the aortic phase contrast velocity-encoded cine images (55,77) (Figure 5). This method is highly reproducible and considered robust as it is not affected by the direction or eccentricity of the regurgitant jet, is not affected by the presence of aortic regurgitation and makes no hemodynamic or LV geometry assumptions, as is often the case in echocardiography (57,70,82). This CMR (volumetric) method was also recently found to have the highest diagnostic value to detect significant MR with an area under the curve (AUC) of 0.98, followed by 3D-echo (AUC =0.96), 2D-echo (AUC =0.90), and CMR (phase contrast; AUC =0.83) (83). - Difference between LV stroke volume and RV stroke volume.
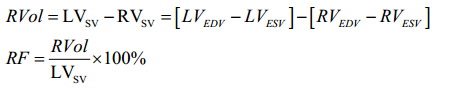
This technique is more prone to error and fails in the context of multiple valvular lesions (55). The calculation of the right ventricle stroke volume is also less reproducible due to the extensive trabeculation of the right ventricle.

Quantitative assessment of MR severity
Grading of severity
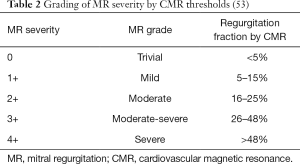
Reference ranges for MR quantification are yet to be as firmly established as those for echocardiography, however, reference ranges for values acquired via quantitative techniques are outlined in Table 2 (53). Myerson et al. found that progression to symptoms and need for MV surgery were seen with a RF of >40% (63). Whilst echocardiography remains the first-line modality for assessment of valvular regurgitation, CMR is increasingly used due to its ability to provide absolute quantitation of both mitral RVol and RF.
Concordance between Echo and CMR
There are a paucity of comparative studies between echocardiography and CMR, and the majority have shown a modest concordance in the qualitative or quantitative evaluation of MR (29,53,56,57,70,84). The latest study demonstrating a modest correlation for RVol/RF parameters has utilised the volumetric PW-Doppler flow quantitation (29). Contrary to above, a prospective multicentre study by Uretsky et al. found that compared to CMR, echocardiographic grading of MR severity was higher and 2D-PISA-derived RVols were larger (70). This discordance was particularly marked in patients who were referred for MV surgery based on the current ACC/AHA recommendations. Amongst the patients referred, approximately two-thirds did not have severe MR by CMR. A tight correlation was found between the RVol calculated using CMR and the degree of LV negative remodelling post-MV surgery, suggesting that RVol by CMR is more accurate than PISA-based RVol by echocardiography. Furthermore, there was no relationship between the PISA-derived RVol (echo) and the degree of LV negative remodelling post-surgery.
In 2016, a retrospective analysis of asymptomatic patients with moderate-severe MR by echocardiography followed patients for a mean duration of 2.5±1.9 years for progression to an indication for MV surgery (63). Patients who did not progress to an indication for surgery and those who did both had mean RVol by echocardiography in the severe range (74±74 vs. 89±36 mL). By CMR, those who did not progress to an indication for MV surgery had lower mean RVol than those who did progress (39±20 vs. 66±24 mL). In this study, RVol by CMR had an AUC of 0.80 for determining which patients would develop an indication for MV surgery. A cut-off of CMR-derived RVol of 55 mL differentiated those who progressed to an indication for surgery from those who did not. However, a cut-off of an EROA of 0.4 cm2 by echo could not differentiate these two groups. These findings have emphasised the predictive value of CMR quantitative parameters in patients with MR. It is also important to note that although the methods for determining severity of MR by echocardiography differ amongst the studies, the method for CMR has been consistent, highlighting the consensus of a single reproducible method for quantifying MR by CMR. The advantages and limitations of each imaging modality in the assessment of MR are summarised in Table 3.
Cardiac CT
Multi-slice Cardiac CT can be particularly useful in the pre-operative setting as it provides complementary information on the feasibility and safety of MV repair or replacement. In addition to evaluating the extent of MV annulus calcification (85), cardiac CT can provide detailed measurements of the MV geometry and assess the angle in between the anterior MV and LV outflow track to aid pre-procedural planning; thus reducing the risk of LV outflow tract obstruction during newer transcatheter techniques of MV replacements (85,86,88). The use of cardiac CT also allows the simultaneous visualization of the cardiac arterial and venous systems, and cardiac anatomy which can further aid the planning of percutaneous MV repair (13). Although cardiac CT with cine imaging can reliably detect and localise segmental leaflet prolapse, this is not routinely performed due to the high radiation dose required (89). Similarly, whilst cardiac CT is particularly useful in excluding coronary artery disease (high negative predictive value in patients who are at low risk of atherosclerosis), its routine use for this in the setting of valvular heart disease is not yet recommended.
In terms of assessing MR severity by cardiac CT, two studies have demonstrated that CT-derived AROA correlates well with EROA measured by echocardiography (89,90). Quantitative RVol can be generated as the difference between the calculated stroke volume of the left and the right ventricle and has been shown to have a good correlation with the RVol obtained by CMR (91). An important caveat is that this technique is not be feasible in the presence of other valve dysfunction. Cardiac CT could however be an alternative for patients with poor echo imaging when CMR is contra-indicated. Whilst routine assessment with cardiac CT is not yet recommended, its role might increase as radiation and contrast doses decrease in the future.
Conclusions
As each imaging modality has its intrinsic advantages and limitations, an integrated multimodality imaging approach is essential for a comprehensive assessment of MR. Although echocardiography is widely accessible and offers excellent morphological and functional information, it is limited by its suboptimal reproducibility in severity assessment and in its evaluation of secondary MR. CMR is highly accurate in the quantitation of MR severity, and should be considered in those with eccentric MR or poor echocardiographic images. Cardiac CT currently serves to provide structural information of MV for novel transcatheter techniques. The choice of imaging modality should be individualised on a case-by-case basis such that each technique is used to its best advantage.
Future directions
Recent developments in the assessment of MR with potential future value include (I) the ‘average pixel intensity’ (API) method (92), a novel digital quantification of the CW pixel intensity in grading MR severity; (II) 4D-flow CMR (93), which allows correction for MV motion; (III) real-time 3D TOE-based 4-dimensional MV models (94), which allow excellent morphological visualization and a comprehensive quantitative analysis of MV annulus and leaflets during the entire cardiac cycle; and (IV) 3D printing of MV (95,96), where the anatomy of a patient-specific MV can be accurately modeled to improve pre-operative planning of complex surgical interventions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on valvular heart disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- De Bonis M, Maisano F, Canna G, La , Alfieri O. Treatment and management of mitral regurgitation. Nat Rev Cardiol 2011;9:133-46. [Crossref] [PubMed]
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [Crossref] [PubMed]
- Grayburn PA, Roberts BJ, Aston S, Anwar A, Hebeler RF Jr, Brown DL, Mack MJ. Mechanism and severity of mitral regurgitation by transesophageal echocardiography in patients referred for percutaneous valve repair. Am J Cardiol 2011;108:882-7. [Crossref] [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. ESC Scientific Document Group 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [Crossref] [PubMed]
- Irvine T, Li XK, Sahn DJ, Kenny A. Assessment of mitral regurgitation. Heart 2002;88 Suppl 4:iv11-9. [Crossref] [PubMed]
- Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL. European Association of Echocardiography. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [Crossref] [PubMed]
- O'Gara P, Sugeng L, Lang R, Sarano M, Hung J, Raman S, Fischer G, Carabello B, Adams D, Vannan M. The Role of Imaging in Chronic Degenerative Mitral Regurgitation. JACC Cardiovasc Imaging 2008;1:221-37. [Crossref] [PubMed]
- Grayburn PA, Fehske W, Omran H, Brickner ME, Lüderitz B. Multiplane transesophageal echocardiographic assessment of mitral regurgitation by Doppler color flow mapping of the vena contracta. Am J Cardiol 1994;74:912-7. [Crossref] [PubMed]
- Heinle SK, Hall SA, Brickner ME, Willett DL, Grayburn PA. Comparison of vena contracta width by multiplane transesophageal echocardiography with quantitative Doppler assessment of mitral regurgitation. Am J Cardiol 1998;81:175-9. [Crossref] [PubMed]
- Zhou X, Jones M, Shiota T, Yamada I, Teien D, Sahn DJ. Vena contracta imaged by Doppler color flow mapping predicts the severity of eccentric mitral regurgitation better than color jet area: A chronic animal study. J Am Coll Cardiol 1997;30:1393-8. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Enriquez-Sarano M, Bailey KR, Seward JB, Tajik AJ, Krohn MJ, Mays JM. Quantitative Doppler assessment of valvular regurgitation. Circulation 1993;87:841-8. [Crossref] [PubMed]
- Van de Heyning CM, Magne J, Vrints CJ, Piérard L, Lancellotti P. The role of multi-imaging modality in primary mitral regurgitation. Eur Heart J Cardiovasc Imaging 2012;13:139-51. [Crossref] [PubMed]
- Biner S, Rafique A, Rafii F, Tolstrup K, Noorani O, Shiota T, Gurudevan S, Siegel RJ. Reproducibility of Proximal Isovelocity Surface Area, Vena Contracta, and Regurgitant Jet Area for Assessment of Mitral Regurgitation Severity. JACC Cardiovasc Imaging 2010;3:235-43. [Crossref] [PubMed]
- Thavendiranathan P, Phelan D, Collier P, Thomas JD, Flamm SD, Marwick TH. Quantitative assessment of mitral regurgitation: How best to do it. Vol. 5 JACC Cardiovasc Imaging 2012;5:1161-75. [Crossref] [PubMed]
- Enriquez-Sarano M, Miller FA, Hayes SN, Bailey KR, Tajik AJ, Seward JB. Effective mitral regurgitant orifice area: Clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol 1995;25:703-9. [Crossref] [PubMed]
- Pu M, Prior DL, Fan X, Asher CR, Vasquez C, Griffin BP, Thomas JD. Calculation of mitral regurgitant orifice area with use of a simplified proximal convergence method: Initial clinical application. J Am Soc Echocardiogr 2001;14:180-5. [Crossref] [PubMed]
- Buck T, Plicht B, Kahlert P, Schenk IM, Hunold P, Erbel R. Effect of Dynamic Flow Rate and Orifice Area on Mitral Regurgitant Stroke Volume Quantification Using the Proximal Isovelocity Surface Area Method. J Am Coll Cardiol 2008;52:767-78. [Crossref] [PubMed]
- Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: Physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol 1999;33:538-45. [Crossref] [PubMed]
- Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet 2005;365:507-18. [Crossref] [PubMed]
- Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral Annulus Calcification. J Am Coll Cardiol 2015;66:1934-41. [Crossref] [PubMed]
- Rokey R, Sterling LL, Zoghbi WA, Sartori MP, Limacher MC, Kuo LC, Quinones MA. Determination of regurgitant fraction in isolated mitral or aortic regurgitation by pulsed Doppler two-dimensional echocardiography. J Am Coll Cardiol 1986;7:1273-8. [Crossref] [PubMed]
- Lopez-Mattei JC, Ibrahim H, Shaikh KA, Little SH, Shah DJ, Maragiannis D, Zoghbi WA. Comparative Assessment of Mitral Regurgitation Severity by Transthoracic Echocardiography and Cardiac Magnetic Resonance Using an Integrative and Quantitative Approach. Am J Cardiol 2016;117:264-70. [Crossref] [PubMed]
- Grayburn PA, Carabello B, Hung J, Gillam LD, Liang D, Mack MJ, McCarthy PM, Miller DC, Trento A, Siegel RJ. Defining “severe” secondary mitral regurgitation: Emphasizing an integrated approach. J Am Coll Cardiol 2014;64:2792-801. [Crossref] [PubMed]
- Agricola E, Bombardini T, Oppizzi M, Margonato A, Pisani M, Melisurgo G, Picano E. Usefulness of latent left ventricular dysfunction assessed by Bowditch Treppe to predict stress-induced pulmonary hypertension in minimally symptomatic severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 2005;95:414-7. [Crossref] [PubMed]
- Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, Ferlito M, Tafanelli L, Bursi F, Trojette F, Branzi A, Habib G, Modena MG, Enriquez-Sarano M. MIDA Investigators. Survival Implication of Left Ventricular End-Systolic Diameter in Mitral Regurgitation Due to Flail Leaflets. A Long-Term Follow-Up Multicenter Study. J Am Coll Cardiol 2009;54:1961-8. [Crossref] [PubMed]
- Enriquez-Sarano M, Tajik AJ, Schaff H V., Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 1994;90:830-7. [Crossref] [PubMed]
- Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, Edvardsen T, Garbi M, Ha JW, Kane GC, Kreeger J, Mertens L, Pibarot P, Picano E, Ryan T, Tsutsui JM, Varga A. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2016;17:1191-229. [Crossref] [PubMed]
- O’Connor K, Lancellotti P, Pierard LA, Piérard LA. Stress Doppler echocardiography in valvular heart diseases: utility and assessment. Future Cardiol 2010;6:611-25. [Crossref] [PubMed]
- Leung DY, Griffin BP, Stewart WJ, Cosgrove DM, Thomas JD, Marwick TH. Left ventricular function after valve repair for chronic mitral regurgitation: predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J Am Coll Cardiol 1996;28:1198-205. [Crossref] [PubMed]
- Picano E, Pibarot P, Lancellotti P, Monin JL, Bonow RO. The Emerging Role of Exercise Testing and Stress Echocardiography in Valvular Heart Disease. J Am Coll Cardiol 2009;54:2251-60. [Crossref] [PubMed]
- Magne J, Lancellotti P, Piérard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol 2010;56:300-9. [Crossref] [PubMed]
- Enriquez-Sarano M, Freeman WK, Tribouilloy CM, Orszulak TA, Khandheria BK, Seward JB, Bailey KR, Tajik AJ. Functional anatomy of mitral regurgitation. J Am Coll Cardiol 1999;34:1129-36. [Crossref] [PubMed]
- Shah PM. Current concepts in mitral valve prolapse-Diagnosis and management. J Cardiol 2010;56:125-33. [Crossref] [PubMed]
- Chambers JB, Garbi M, Nieman K, Myerson S, Pierard LA, Habib G, Zamorano JL, Edvardsen T, Lancellotti P. This document was reviewed by members of the 2014—16 EACVI Scientific Documents Committee:, Delgado V, Cosyns B, Donal E, Dulgheru R, Galderisi M, Lombardi M, Muraru D, Kauffmann P, Cardim N, Haugaa K, Rosenhek R. Appropriateness criteria for the use of cardiovascular imaging in heart valve disease in adults: a European Association of Cardiovascular Imaging report of literature review and current practice. Eur Heart J Cardiovasc Imaging 2017;18:489-98. [Crossref] [PubMed]
- Macnab A, Jenkins NP, Bridgewater BJ, Hooper TL, Greenhalgh DL, Patrick MR, Ray SG. Three-dimensional echocardiography is superior to multiplane transoesophageal echo in the assessment of regurgitant valve morphology. Eur J Echocardiogr 2004;5:212-22. [Crossref] [PubMed]
- La Canna G, Arendar I, Maisano F, Monaco F, Collu E, Benussi S, De Bonis M, Castiglioni A, Alfieri O. Real-time three-dimensional transesophageal echocardiography for assessment of mitral valve functional anatomy in patients with prolapse-related regurgitation. Am J Cardiol 2011;107:1365-74. [Crossref] [PubMed]
- Marsan NA, Westenberg JJ, Ypenburg C, Delgado V, van Bommel RJ, Roes SD, Nucifora G, van der Geest RJ, de Roos A, Reiber JC, Schalij MJ, Bax JJ. Quantification of Functional Mitral Regurgitation by Real-Time 3D Echocardiography. Comparison With 3D Velocity-Encoded Cardiac Magnetic Resonance. JACC Cardiovasc Imaging 2009;2:1245-52. [Crossref] [PubMed]
- Choi J, Heo R, Hong GR, Chang HJ, Sung JM, Shin SH, Cho IJ, Shim CY, Chung N. Differential effect of 3-dimensional color Doppler echocardiography for the quantification of mitral regurgitation according to the severity and characteristics. Circ Cardiovasc Imaging 2014;7:535-44. [Crossref] [PubMed]
- Matsumura Y, Fukuda S, Tran H, Greenberg NL, Agler DA, Wada N, Toyono M, Thomas JD, Shiota T. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color Doppler echocardiography: Difference between functional mitral regurgitation and prolapse regurgitation. Am Heart J 2008;155:231-8. [Crossref] [PubMed]
- Matsumura Y, Saracino G, Sugioka K, Tran H, Greenberg NL, Wada N, Toyono M, Fukuda S, Hozumi T, Thomas JD, Yoshikawa J, Yoshiyama M, Shiota T. Determination of Regurgitant Orifice Area with the Use of a New Three-Dimensional Flow Convergence Geometric Assumption in Functional Mitral Regurgitation. J Am Soc Echocardiogr 2008;21:1251-6. [Crossref] [PubMed]
- Altiok E, Hamada S, van Hall S, Hanenberg M, Dohmen G, Almalla M, Grabskaya E, Becker M, Marx N, Hoffmann R. Comparison of Direct Planimetry of Mitral Valve Regurgitation Orifice Area by Three-Dimensional Transesophageal Echocardiography to Effective Regurgitant Orifice Area Obtained by Proximal Flow Convergence Method and Vena Contracta Area Determined by Color. Am J Cardiol 2011;107:452-8. [Crossref] [PubMed]
- Chandra S, Salgo IS, Sugeng L, Weinert L, Settlemier SH, Mor-Avi V, Lang RM. A three-dimensional insight into the complexity of flow convergence in mitral regurgitation: adjunctive benefit of anatomic regurgitant orifice area. Am J Physiol Heart Circ Physiol 2011;301:H1015-24. [Crossref] [PubMed]
- Thomas N, Unsworth B, Ferenczi EA, Davies JE, Mayet J, Francis DP. Intraobserver variability in grading severity of repeated identical cases of mitral regurgitation. Am Heart J 2008;156:1089-94. [Crossref] [PubMed]
- Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J 2000;21:1387-96. [Crossref] [PubMed]
- Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:7. [Crossref] [PubMed]
- Gelfand EV, Hughes S, Yeon S. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson 2006;8:503-7. [Crossref] [PubMed]
- Gelfand EV, Manning WJ. Assessment of valvular heart disease with cardiovascular magnetic resonance. Indian J Radiol Imaging 2007;17:120-32. [Crossref]
- Kon MWS, Myerson SG, Moat NE, Pennell DJ. Quantification of regurgitant fraction in mitral regurgitation by cardiovascular magnetic resonance: comparison of techniques. J Heart Valve Dis 2004;13:600-7. [PubMed]
- Aplin M, Kyhl K, Bjerre J, Ihlemann N, Greenwood JP, Plein S, Uddin A, Tønder N, Høst NB, Ahlström MG, Hove J, Hassager C, Iversen K, Vejlstrup NG, Lav Madsen P. Cardiac remodelling and function with primary mitral valve insufficiency studied by magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2016;17:863-70. [Crossref] [PubMed]
- Cawley PJ, Hamilton-Craig C, Owens DS, Krieger EV, Strugnell WE, Mitsumori L, D'Jang CL, Schwaegler RG, Nguyen KQ, Nguyen B, Maki JH, Otto CM. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 2013;6:48-57. [Crossref] [PubMed]
- Barone-Rochette G, Piérard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, Pouleur AC, Vancraeynest D, Pasquet A, Vanoverschelde JL, Gerber BL. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol 2014;64:144-54. [Crossref] [PubMed]
- Nigri M, Azevedo CF, Rochitte CE, Schraibman V, Tarasoutchi F, Pommerantzeff PM, Brandão CM, Sampaio RO, Parga JR, Avila LF, Spina GS, Grinberg M. Contrast-enhanced magnetic resonance imaging identifies focal regions of intramyocardial fibrosis in patients with severe aortic valve disease: Correlation with quantitative histopathology. Am Heart J 2009;157:361-8. [Crossref] [PubMed]
- Chuang ML, Manning WJ. Left Ventricular Hypertrophy and Excess Cardiovascular Mortality. Is Late Gadolinium Enhancement the Imaging Link? J Am Coll Cardiol 2009;53:292-4. [Crossref] [PubMed]
- Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol 2014;114:1083-8. [Crossref] [PubMed]
- Van De Heyning CM, Magne J, Piérard LA, Bruyère PJ, Davin L, De Maeyer C, Paelinck BP, Vrints CJ, Lancellotti P. Late gadolinium enhancement CMR in primary mitral regurgitation. Eur J Clin Invest 2014;44:840-7. [Crossref] [PubMed]
- Myerson SG, d'Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, Prendergast B, Greenwood JP, Karamitsos TD, Neubauer S. Determination of Clinical Outcome in Mitral Regurgitation With Cardiovascular Magnetic Resonance Quantification. Circulation 2016;133:2287-96. [Crossref] [PubMed]
- Ripley DP, Musa TA, Dobson LE, Plein S, Greenwood JP. Cardiovascular magnetic resonance imaging: what the general cardiologist should know. Heart 2016;102:1589-603. [Crossref] [PubMed]
- Expert Panel on MR Safety1, Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr, Froelich JW, Gimbel JR, Gosbee JW, Kuhni-Kaminski E, Larson PA, Lester JW Jr, Nyenhuis J, Schaefer DJ, Sebek EA, Weinreb J, Wilkoff BL, Woods TO, Lucey L, Hernandez D. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 2013;37:501-30. [PubMed]
- Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular Magnetic Resonance Characterization of Mitral Valve Prolapse. Jacc-Cardiovascular Imaging 2008;1:294-303. [Crossref] [PubMed]
- Chinitz JS, Chen D, Goyal P, Wilson S, Islam F, Nguyen T, Wang Y, Hurtado-Rua S, Simprini L, Cham M, Levine RA, Devereux RB, Weinsaft JW. Mitral apparatus assessment by delayed enhancement CMR: Relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging 2013;6:220-34. [Crossref] [PubMed]
- Stork A, Franzen O, Ruschewski H, Detter C, Müllerleile K, Bansmann PM, Adam G, Lund GK. Assessment of functional anatomy of the mitral valve in patients with mitral regurgitation with cine magnetic resonance imaging: Comparison with transesophageal echocardiography and surgical results. Eur Radiol 2007;17:3189-98. [Crossref] [PubMed]
- Cawley PJ, Maki JH, Otto CM. Cardiovascular magnetic resonance imaging for valvular heart disease. Technique and validation. Circulation 2009;119:468-78. [Crossref] [PubMed]
- Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, Gurram S, Jain K, Subero M, Jang JJ, Cohen R, Wolff SD. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: A prospective multicenter trial. J Am Coll Cardiol 2015;65:1078-88. [Crossref] [PubMed]
- Buchner S, Debl K, Poschenrieder F, Feuerbach S, Riegger GA, Luchner A, Djavidani B. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging 2008;1:148-55. [Crossref] [PubMed]
- Chatzimavroudis GP, Oshinski JN, Franch RH, Walker PG. Yoganathan a P, Pettigrew RI. Evaluation of the precision of magnetic resonance phase velocity mapping for blood flow measurements. J Cardiovasc Magn Reson 2001;3:11-9. [Crossref] [PubMed]
- Aurigemma G, Reichek N, Schiebler M, Axel L. Evaluation of mitral regurgitation by cine magnetic resonance imaging. Am J Cardiol 1990;66:621-5. [Crossref] [PubMed]
- Pflugfelder PW, Sechtem UP, White RD, Cassidy MM, Schiller NB, Higgins CB. Noninvasive evaluation of mitral regurgitation by analysis of left atrial signal loss in cine magnetic resonance. Am Heart J 1989;117:1113-9. [Crossref] [PubMed]
- Buchner S, Poschenrieder F, Hamer OW, Jungbauer C, Resch M, Birner C, Fellner C, Riegger GA, Stroszczynski C, Djavidani B, Debl K, Luchner A. Direct visualization of regurgitant orifice by CMR reveals differential asymmetry according to etiology of mitral regurgitation. JACC Cardiovasc Imaging 2011;4:1088-96. [Crossref] [PubMed]
- Hundley WG, Li HF, Willard JE, Landau C, Lange RA, Meshack BM, Hillis LD, Peshock RM. Magnetic resonance imaging assessment of the severity of mitral regurgitation: Comparison with invasive techniques. Circulation 1995;92:1151-8. [Crossref] [PubMed]
- Fujita N, Chazouilleres AF, Hartiala JJ, O'Sullivan M, Heidenreich P, Kaplan JD, Sakuma H, Foster E, Caputo GR, Higgins CB. Quantification of mitral regurgitation by velocity-encoded cine nuclear magnetic resonance imaging. J Am Coll Cardiol 1994;23:951-8. [Crossref] [PubMed]
- Lopez-Mattei JC, Shah DJ. The role of cardiac magnetic resonance in valvular heart disease. Methodist Debakey Cardiovasc J 2013;9:142-8. [Crossref] [PubMed]
- Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: part II. J Cardiovasc Magn Reson 2012;14:66. [Crossref] [PubMed]
- Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging. Magn Reson Q 1991;7:229-54. [PubMed]
- Krieger EV, Lee J, Branch KR, Hamilton-Craig C. Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart 2016;102:1864-70. [Crossref] [PubMed]
- Uretsky S, Supariwala A, Nidadovolu P, Khokhar SS, Comeau C, Shubayev O, Campanile F, Wolff SD. Quantification of left ventricular remodeling in response to isolated aortic or mitral regurgitation. J Cardiovasc Magn Reson 2010;12:32. [Crossref] [PubMed]
- Le Goffic C, Toledano M, Ennezat PV, Binda C, Castel AL, Delelis F, Graux P, Tribouilloy C, Maréchaux S. Quantitative Evaluation of Mitral Regurgitation Secondary to Mitral Valve Prolapse by Magnetic Resonance Imaging and Echocardiography. Am J Cardiol 2015;116:1405-10. [Crossref] [PubMed]
- Kizilbash AM, Hundley WG, Willett DL, Franco F, Peshock RM, Grayburn PA. Comparison of quantitative doppler with magnetic resonance imaging for assessment of the severity of mitral regurgitation. Am J Cardiol 1998;81:792-5. [Crossref] [PubMed]
- Manghat NE, Rachapalli V, Van Lingen R, Veitch AM, Roobottom CA, Morgan-Hughes GJ. Imaging the heart valves using ECG-gated 64-detector row cardiac CT. Br J Radiol 2008;81:275-90. [Crossref] [PubMed]
- Shanks M, Delgado V, Ng AC, van der Kley F, Schuijf JD, Boersma E, van de Veire NR, Nucifora G, Bertini M, de Roos A, Kroft L, Schalij MJ, Bax JJ. Mitral valve morphology assessment: Three-dimensional transesophageal echocardiography versus computed tomography. Ann Thorac Surg 2010;90:1922-9. [Crossref] [PubMed]
- Thavendiranathan P, Phelan D, Thomas JD, Flamm SD, Marwick TH. Quantitative assessment of mitral regurgitation: Validation of new methods. J Am Coll Cardiol 2012;60:1470-83. [Crossref] [PubMed]
- Murphy DJ, Ge Y, Don CW, Keraliya A, Aghayev A, Morgan R, Galper B, Bhatt DL, Kaneko T, Di Carli M, Shah P, Steigner M, Blankstein R. Use of cardiac computerized tomography to predict neo-left ventricular outflow tract obstruction before transcatheter mitral valve replacement. J Am Heart Assoc 2017.6. [PubMed]
- Alkadhi H, Wildermuth S, Bettex DA, Plass A, Baumert B, Leschka S, Desbiolles LM, Marincek B, Boehm T. Mitral regurgitation: quantification with 16-detector row CT--initial experience. Radiology 2006;238:454-63. [Crossref] [PubMed]
- Vural M, Ucar O, Celebi OO, Cicekcioglu H, Durmaz HA, Selvi NA, Koparal S, Aydogdu S. Evaluation of effective regurgitant orifice area of mitral valvular regurgitation by multislice cardiac computed tomography. J Cardiol 2010;56:236-9. [Crossref] [PubMed]
- Guo YK, Yang ZG, Ning G, Rao L, Dong L, Pen Y, Zhang TM, Wu Y, Zhang XC, Wang QL. Isolated mitral regurgitation: quantitative assessment with 64-section multidetector CT--comparison with MR imaging and echocardiography. Radiology 2009;252:369-76. [Crossref] [PubMed]
- El Haddad M, De Backer T, De Buyzere M, Devos D, Swillens A, Segers P, Timmermans F. Grading of mitral regurgitation based on intensity analysis of the continuous wave Doppler signal. Heart 2017;103:190-7. [Crossref] [PubMed]
- Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll CJ, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015;17:72. [Crossref] [PubMed]
- Noack T, Mukherjee C, Kiefer P, Emrich F, Vollroth M, Ionasec RI, Voigt I, Houle H, Ender J, Misfeld M, Mohr FW, Seeburger J. Four-dimensional modelling of the mitral valve by real-time 3D transoesophageal echocardiography: Proof of concept. Interact Cardiovasc Thorac Surg 2015;20:200-8. [Crossref] [PubMed]
- Ginty OK, Moore JM, Xu Y, Xia W, Fujii S, Bainbridge D, Peters TM, Kiaii BB, Chu MWA. Dynamic Patient-Specific Three-Dimensional Simulation of Mitral Repair: Can We Practice Mitral Repair Preoperatively? Innovations (Phila) 2018;13:11-22. [Crossref] [PubMed]
- Ginty O, Moore J, Peters T, Bainbridge D. Modeling Patient-Specific Deformable Mitral Valves. J Cardiothorac Vasc Anesth 2017. Epub ahead of print. [Crossref] [PubMed]