Quantitative contrast ratio comparison between T1 (TSE at 1.5T, FLAIR at 3T), magnetization prepared rapid gradient echo and subtraction imaging at 1.5T and 3T
Introduction
The main goal in imaging is to create contrast between anatomic and pathologic structures. This is used throughout radiology whether the technique is plain film X-ray, CT, MRI, ultrasound or nuclear medicine. In particular, MRI uses a wide array of techniques ranging from the commonly used T1 and T2 sequences to perfusion, diffusion tensor imaging and functional MRI. From the earlier days intravenous contrast agents have been used to enhance different pathologies generating improved contrast between them and adjacent structures. Contrast agents are particularly important in the detection and characterization of intracranial pathology with particular emphasis on neoplastic disease, infectious and inflammatory pathologies and vascular abnormalities. One question is which T1 weighted imaging technique using contrast agent generates the best contrast between the pathology and adjacent tissues, T1 TSE or magnetization prepared rapid gradient echo (MPRAGE) at 1.5T and T1 FLAIR and MPRAGE at 3T. Determining and comparing the contrast to background ratios of these imaging techniques is one of the goals of this research.
In the earliest days of angiography one problem was that the skeleton would degrade the visualization of the blood vessels being injected with contrast. A significant improvement in vessel visualization was introduced by Ziedses des Plantes (1) in 1935 with the use of image subtraction which consisted of first taking a baseline image and then subtracting that image from subsequent images taken while the vessels were being injected with contrast. This was first done manually and then later was available digitally. This has since been copied in a similar fashion with MRA where the baseline images are taken, followed by another set of images timed appropriately such that the IV contrast bolus is within the arteries during the imaging then these two series are subtracted from each other to produce images where the contrast filled blood vessels stand out dramatically from the adjacent tissues improving the quality of the maximum intensity projection (MIP) images. A (2). This feature is commonly available on modern MRI scanners and is also often used with breast imaging. Interestingly using subtraction for other imaging, such as the brain, has only been occasionally mentioned in the literature and is not commonly used in clinical practice. The other purpose of this research is do evaluate the ease and utility of using subtraction imaging and compare the contrast to background ratios using subtraction between T1 TSE and MPRAGE at 1.5T and between T1 FLAIR and MPRAGE at 3T.
Materials and methods
This project was approved by the University of Louisville Institutional Review Board. A total of 27 exams on 25 patients scheduled for brain MRIs for the purpose of lesion localization for either radiation treatment planning or neurosurgery were included in this study. All brain imaging was performed on either a 1.5T Siemens Espree or 3T Siemens Verio MRI systems.
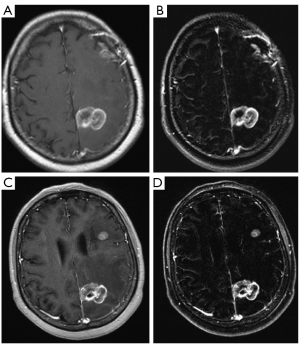
For the 1.5T Siemens Espree all subjects were scanned with pre and post contrast T1 TSE MR technique (TR/TE 400/min, excitations 2, field of view 24 cm, matrix 320×256, slice thickness/gap 5/1) in addition pre and post contrast MPRAGE MR technique (TR/TE 897/4.55, TI 600, FA 150, excitations 1, field of view 35 cm, matrix 512×512, slice thickness/gap 1.5/0) were performed. Digital subtraction was performed for both the T1 TSE imaging as well as the MPRAGE imaging. All images were sent to PACS (Figure 1).
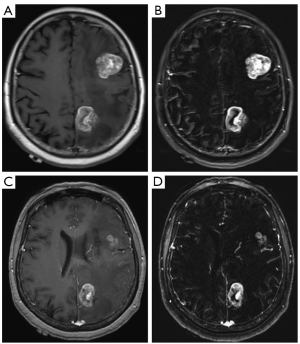
For the 3T Siemens Verio all subjects were scanned with pre and post contrast T1 FLAIR MR technique (TR/TE 2,000/9, TI 860, excitations 1, field of view 22 cm, matrix 240×320, slice thickness/gap 4/1.2) in addition to pre and post contrast MPRAGE MR technique (TR/TE 1,190/2.89, TI 900, FA 9, excitations 1, field of view 35 cm, matrix 512×512, slice thickness/gap 1.5/0) were performed. Digital subtraction was performed for both the T1 FLAIR as well as the MPRAGE imaging. All images were sent to PACS (Figure 2).
Lesion diameter was recorded for every enhancing lesion in every patient. For each of the post contrast sequences and each of the subtraction sequences radiologist determined region of interest (ROI) were placed and mean signal intensity values recorded for each lesion and the contralateral brain. The ROI was placed only over a representative part of each lesion’s enhancing component (i.e. for ring enhancing lesions the ring was used) including only enhancing tissue. Contrast ratio was then calculated using the following equation:
CR = (Ce–Cb)/Cb [1]
(note: Ce, Signal intensity of enhancing lesion)
All data was tabulated and lesion size versus CR graphed for each sequence. For several subtraction sequences the Signal intensity of contralateral brain (Cb) value was zero, in these circumstances the Cb value was arbitrarily given the value of 1 to prevent division by zero and inappropriate magnification of the CR value. Comparison of CR values versus lesion size was performed at 1.5T as T1 TSE vs. T1 TSE subtracted, MPRAGE vs. MPRAGE subtracted, T1 TSE vs. MPRAGE and T1 TSE subtracted vs. MPRAGE subtracted and for the 3T data as T1 FLAIR vs. T1 FLAIR subtracted, MPRAGE vs. MPRAGE subtracted, T1 FLAIR vs. MPRAGE and T1 FLAIR subtracted vs. MPRAGE subtracted both graphically and using the two tailed paired t-test and P-values were generated. Any P-values of 0.05 or less were considered significant.
Since this project is to compare lesion enhancement only patients specifically scheduled for our Varian protocol (used for radiation treatment planning and preoperative planning) were included in the study since these were all expected to have positive findings. A total of 27 consecutive exams on 25 patients were analyzed. This yielded a total of 90 lesions ranging from a minimum of 1 to a maximum of 17 with an average of 3.4 lesions per exam. All lesions seen in each exam were included with one exam excluded due to significant motion artifact.
Results
A total of 90 enhancing brain lesions (35 at 1.5T and 55 at 3T) were utilized. Of these 46 were <5 mm diameter (15 at 1.5T and 31 at 3T). These included 10 patients with metastatic lung cancer, 1 arteriovenous malformation, 2 vestibular schwannomas, 2 malignant melanoma, 1 orbit tumor, 2 presumed meningiomas, 2 metastatic colorectal cancer, 1 metastatic ovarian cancer, 1 pituitary mass, 2 glioblastoma multiforme and 1 metastatic uterine cancer. At 1.5T there was one 2 mm diameter enhancing lesion only seen on the MPRAGE sequences, a 3 mm enhancing lesion only seen on T1 TSE sequences and one 2 mm lesion not seen on subtraction imaging. At 3T all enhancing lesions were seen on all sequences.
At 1.5T taking all lesions into account (Figure 3A) there was a small but statistically significant improvement in contrast ratio for MPRAGE compared to the T1 TSE (P=0.01). However, when using only lesions <5 mm diameter (Figure 3B) there was no statistically significant difference in CR between MPRAGE and T1 TSE sequences (P=0.20). Subtraction provided a marked improvement in CR which was statistically significant for all lesions (T1 TSE vs. subtraction P<0.001, MPRAGE vs. its subtraction P<0.001) which persisted when evaluating only lesions <5 mm diameter (Figure 3) (T1 TSE vs. its subtraction P=0.02, MPRAGE vs. its subtraction P<0.001). Comparison of subtraction between T1 TSE and MPRAGE yielded no statistically significant difference whether using all lesions (P=0.11) or using only lesions <5 mm diameter (P=0.37).
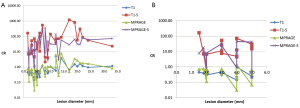
At 3T taking all lesions into account (Figure 4A) there was a small but statistically significant improvement in CR for T1 FLAIR compared to MPRAGE (P=0.005). However, when taking only lesions <5 mm (Figure 4B) there was no statistically significant difference (P=0.60). Taking all lesions into account subtraction provided a statistically significant marked improvement in CR for both T1 FLAIR and MPRAGE (P<0.001 for both T1 FLAIR vs. its subtraction and MPRAGE vs. its subtraction) which persisted when comparing only lesions <5 mm diameter (Figure 4) (P<0.001 for both). Comparison between subtraction of T1 FLAIR and MPRAGE yielded a small but statistically significant greater CR for T1 FLAIR when using all lesions (P=0.03) but was not statistically significant when using only lesions <5 mm diameter (P=0.11).

Discussion
The presence of brain metastases and their number is a significant prognostic factor particularly for patients with non-small cell lung cancer, small cell lung cancer, melanoma and renal cell carcinoma (3). The first goal of any imaging exam is the detection of abnormality. Without detection appropriate treatment may be delayed. In order to improve lesion detection various MRI imaging sequences as well as several contrast agents have been developed. There have been several articles discussing the advantages and disadvantages of different MRI sequences. In 1990 Cherryman et al. compared T1 spin echo (SE) with fast low angle shot (FLASH) 90o and found SE to be better in detection of brain metastases (4). In 1992 Mirowitz et al. compared T1 SE with 3D Fourier transform gradient recalled acquisition in steady state (GRASS) gradient echo (GRE) technique finding no significant difference in the conspicuity of enhancing lesions between the techniques (5). In 1992 Brant-Zawadzki et al. compared T1 SE with MPRAGE finding that while image quality and gray-white matter contrast was superior with MPRAGE, lesion enhancement was equal (15 of 19 patients) or better (4 of 19 patients) with T1 SE with one patient having 2 enhancing lesions seen on T1 SE but not on MPRAGE (6). In 1996 Li et al. compared T1 SE with 3D GRE finding that T1 SE had better signal to noise compared to GRE due to its thicker slices but that small lesions were better visualized in the thin 3D GRE than the thicker SE sequences (7). In 2008 Furutani et al. compared T1 SE to 3-dimensional fast SPoiled gradient recalled acquisition in the steady state (3DFSPGR) at 3T finding that under the same conditions enhancement of 3DFSPGR was less than that of the SE images but not significantly whereas the thin slice 3DFSPGR provided better detectability than SE finding 81 lesions to 79 for SE (8). One parameter that none of these studies used for their comparison was lesion size. This research takes lesion size into account finding that while at for all lesions the MPRAGE had a statistically significant albeit small increase in the contrast to background at 1.5T at 3T the T1 FLAIR was superior. However, for the lesions 5 mm or less there was no significant difference between either T1 TSE and MPRAGE at 1.5T or T1 FLAIR and MPRAGE at 3T.
While Ziedses des Plantes introduced subtraction to X-rays in 1935 (1) one of the earliest forays into subtraction with MRI was performed by Suto et al using Gd-DTPA to better demonstrate enhancement in tissues with fat and bone marrow (9). Since then subtraction has been used for many different body regions as shown by Lee et al. in 1996 (10), as a method to reduce contrast dosage by Chan et al. in 2002 (11), to improve detection of brain lesions in general by Melhem et al. in 1999 (12), by Hanna et al. in 1991 to improve characterization of hemorrhagic lesions as potentially malignant or benign (13) and by Algin et al. (14) and Gavra et al. (15) to demonstrate improved detection of enhancing lesions in multiple sclerosis using subtraction and magnetization transfer imaging. However, none of these studies used lesion size as a significant parameter nor included 3T. This study shows that subtraction of pre- and post contrast imaging regardless of technique clearly demonstrates significantly improved contrast to background ratios for any size metastatic brain lesion at both 1.5T and 3T.
Most studies relied on the qualitative evaluation of lesion detection which is necessarily observer dependent. Some used a quantitative comparison using the formula Contrast to noise ratio (CNR) = SNR lesion – SNR WM which does not take into account the affect of the overall signal level in the conspicuity of the lesion. A qualitative example would be the visibility of a dim flashlight at night versus a brighter flashlight during the day. A quantitative example is CNR =110–10=100 is considered the same as CNR =1100–1000=100. However, normalizing the formula to the contralateral normal brain using:
CR = (SI lesion – SI WM)/SI WM [2]
Provides balance which using the quantitative example performed previously now yields CR = (110–10)/10=10 and CR = (1100–1000)/1000=0.1. This also better conforms to Weber’s Law which states that the ability to detect incremental differences increases in proportion to the background which was also deduced by Bernoulli (16,17). This is particularly pertinent since the crux of the argument is which sequence better allows detection of just noticeable differences in contrast, i.e. the detection of small lesions since the detection of large enhancing lesions is usually not a problem. The data demonstrates that including the larger lesions results in a mild but statistically significant improvement in CR for MPRAGE compared to T1 TSE at 1.5T, for T1 FLAIR compared to MPRAGE at 3T and for T1 FLAIR subtraction compared to MPRAGE subtraction at 3T. However, when including only lesions 5 mm or less there is no statistically significant difference between these sequences with the only comparisons that remain significant being comparison between subtracted and nonsubtracted values. Our data indicate that there should be no clinically significant difference between the non-subtraction techniques for large lesions based on their usual ease of detection or for small lesions based on their similar CR. However, image subtraction provides a clear improvement in CR which holds for each of the non-subtracted techniques regardless of lesion size. Image subtraction is a simple technique that can be automated and has been shown to be helpful in evaluation of hemorrhagic lesions (13). Disadvantages for subtraction are its susceptibility to motion although this is somewhat mitigated by the relative homogeneity of the brain signal. Additionally, the significant enhancement of the brain leptomeninges and sulcal vasculature may reduce the detection of small cortical lesions or mask subtle pathologic leptomeningeal enhancement although this was not assessed with this exam as none of the patient’s demonstrated abnormal leptomeningeal enhancement on any imaging sequence.
Limitations of this study are the relatively small sample size and not performing a qualitative analysis of lesion detection. However, the second limitation is less pertinent since this study’s goal was to provide an objective rather than subjective assessment. Additionally, not all of the lesions were pathologically proven but this is not considered to be a significant limitation since this study is focused on lesion detection regardless of the pathologic diagnosis.
Conclusions
Detection of pathology is one of the key purposes of imaging. Detection of large masses is not usually a problem but the detection of small lesions can be more challenging partly related to their small size compounded by their reduced enhancement. Our data indicate that for small lesions at 1.5T there is no significant difference in CR between T1 TSE and MPRAGE despite the MPRAGE having the advantage of much thinner slices and a higher matrix. At 3T there was also no significant difference between T1 FLAIR and MPRAGE again despite the higher resolution used for MPRAGE. However, subtraction provided a markedly improved CR for all lesions at 1.5T and 3T for all of the sequences. Subtraction should be considered for clinical use to improve detection of small or subtle enhancing lesions.
Acknowledgements
Disclosure: This research has been presented as a scientific presentation at the 2012 Annual Meeting of the American Society of Neuroradiology and has been previously submitted to the American Journal of Neuroradiology and Neuroradiology.
References
- Ziedzes des Plantes BG. Eine Röntgenographische Methode zur separaten Abbildung bestimmter Teile des Objekts. Fortschr Röntgenstr 1935;52:69-79.
- Edelman RR, Hesselink JR, Zlatkin MB. Clinical Magnetic Resonance Imaging. Philadelphia: Saunders Elsevier, 2006.
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [PubMed]
- Cherryman G, Golfieri R. Comparison of spin echo T1-weighted and FLASH 90 degrees gadolinium-enhanced magnetic resonance imaging in the detection of cerebral metastases. Br J Radiol 1990;63:712-5. [PubMed]
- Mirowitz SA. Intracranial lesion enhancement with gadolinium: T1-weighted spin-echo versus three-dimensional Fourier transform gradient-echo MR imaging. Radiology 1992;185:529-34. [PubMed]
- Brant-Zawadzki M, Gillan GD, Nitz WR. MP. RAGE: a three-dimensional, T1-weighted, gradient-echo sequence--initial experience in the brain. Radiology 1992;182:769-75. [PubMed]
- Li D, Haacke EM, Tarr RW, et al. Magnetic resonance imaging of the brain with gadopentetate dimeglumine-DTPA: comparison of T1-weighted spin-echo and 3D gradient-echo sequences. J Magn Reson Imaging 1996;6:415-24. [PubMed]
- Furutani K, Harada M, Mawlan M, et al. Difference in enhancement between spin echo and 3-dimensional fast spoiled gradient recalled acquisition in steady state magnetic resonance imaging of brain metastasis at 3-T magnetic resonance imaging. J Comput Assist Tomogr 2008;32:313-9. [PubMed]
- Suto Y, Caner BE, Tamagawa Y, et al. Subtracted synthetic images in Gd-DTPA enhanced MR. J Comput Assist Tomogr 1989;13:925-8. [PubMed]
- Lee VS, Flyer MA, Weinreb JC, et al. Image subtraction in gadolinium-enhanced MR imaging. AJR Am J Roentgenol 1996;167:1427-32. [PubMed]
- Chan JH, Tsui EY, Chan CY, et al. Digital subtraction in gadolinium-enhanced MR imaging of the brain: a method to reduce contrast dosage. Eur Radiol 2002;12:2317-21. [PubMed]
- Melhem ER, Mehta NR. Dynamic T1-weighted spin-echo MR imaging: the role of digital subtraction in the demonstration of enhancing brain lesions. J Magn Reson Imaging 1999;9:503-8. [PubMed]
- Hanna SL, Langston JW, Gronemeyer SA. Value of subtraction images in the detection of hemorrhagic brain lesions on contrast-enhanced MR images. AJNR Am J Neuroradiol 1991;12:681-5. [PubMed]
- Algin O, Hakyemez B, Taşkapilioğlu O, et al. Imaging of active multiple sclerosis plaques: efficiency of contrast-enhanced magnetization transfer subtraction technique. Diagn Interv Radiol 2010;16:106-11. [PubMed]
- Gavra MM, Voumvourakis C, Gouliamos AD, et al. Brain MR post-gadolinium contrast in multiple sclerosis: the role of magnetization transfer and image subtraction in detecting more enhancing lesions. Neuroradiology 2004;46:205-10. [PubMed]
- Guyton AC. eds. Textbook of Medical Physiology. W.B. Philadelphia: Saunders Company, 1991.
- Masin SC, Zudini V, Antonelli M. Early alternative derivations of Fechner’s law. J Hist Behav Sci 2009;45:56-65. [PubMed]


