Longitudinal CT study of sarcopenia due to hepatic failure after living donor liver transplantation
Introduction
The liver plays a major role in metabolism, but when affected by disease may lead to inadequate food intake, abnormal nutrient metabolism and altered digestion and absorption, together with an increased protein catabolism and an increase in protein-energy requirements (1,2). In patients with liver cirrhosis, this nutritional deficiency can cause a decrease in skeletal muscle mass, namely sarcopenia. Sarcopenia is characterized by lower skeletal muscle quantity, higher fat accumulation in the muscle, lower muscle strength, lower physical performance, leading to poor quality of life (QOL) (3-6).
As radiologic imaging analysis provides direct visualization of body and tissue components, its use in body composition and nutritional assessment is specifically valuable for quantifying nutritional deficiency for which traditional measures of nutrition (biochemical markers, body weight, or anthropomorphic measurements) have proven less accurate (7). Especially, computed tomography (CT) scanning provides an exact measure of muscle mass and has proven more accurate than externally measured muscle circumference (8). For example, the psoas muscle area measured using CT images is reported to be a prognostic factor for liver transplantation (LT) recipients; small psoas muscle area is associated with mortality (6,9-11). The dorsal muscle group area is also a useful prognostic factor (12). Furthermore, dorsal muscle group quality (i.e., fat accumulation of the muscle) has been demonstrated to be an indicator of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (13,14).
To the best our knowledge, there are no reports in the literature concerning follow-up evaluations of LT recipients in terms of trunk muscle area and adiposity. Changes in the size of trunk muscle are thought to best reflect log-term chronic illness and systemic health (3,13,15).
The aim of this study is to analyze trunk muscle mass and adiposity change after living donor liver transplantation (LDLT).
Methods
Patients
Thirty-two adult (age ≥18 years) recipients underwent LDLT at Hokkaido University Hospital between January 2009 and December 2013. One recipient was not alive 1 year after LDLT. A total of 31 recipients (14 males, 17 females) of LDLT were therefore enrolled in the study. Each recipient had an abdominal CT exam (dynamic CT or non-dynamic contrast enhanced CT) at pre-LDLT and 1-year follow-up. Table 1 shows the characteristics of the study population and Table 2 shows the recipients’ disease status.

Full table
Full table
Image analysis
Pre- and post-contrast axial CT images were used to carry out image analysis. Muscle areas and quality (fat accumulation of the muscle) were measured by a radiological technologist using a region of interest (ROI) precisely traced with the use of commercially available image analysis software (volume analyzer SYNAPSE VINCENT, Fujifilm Medical Co., Ltd., Tokyo, Japan). All CT images were acquired at 120 kVp and 5 mm slice thickness. We measured muscle area and adiposity using a single slice. Muscle areas were calculated after being normalizing by the square of the height of the patients. Both pre- and post-operative muscle were measured (16).
The bilateral dorsal muscle group area at the lower border of the 12th thoracic vertebra (Th12) divided by the square of the height of the patient was defined as the dorsal muscle group mass index (DMGMI) (mm2/m2) (12). The bilateral psoas muscle area at the upper border of 4th lumber vertebra (L4) divided by the square of the height of the patient was likewise defined as the psoas muscle mass index (PMI) (mm2/m2) (9).
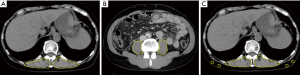
The dorsal muscle group adiposity at the lower border of Th12 was furthermore defined as the intramuscular adipose tissue content (IMAC) following Kitajima et al. (13,14). The density of the dorsal muscle group was measured at the lower border of Th12 using plain CT images. CT values of subcutaneous adipose tissues (SAT) were also measured at four points, keeping away from major vessels using circle ROIs with a diameter of 6 mm. IMAC was calculated as previously described by Kitajima et al.; it was calculated as the mean CT value of the dorsal muscle group (Hounsfield units) divided by the mean CT value of SAT (Hounsfield units), which indicates the degree of steatosis (14). IMAC is normally a negative value. When IMAC is high, more fat infiltration is in the dorsal muscle group, whereas when IMAC is low, there is less fat in the dorsal muscle group. Figure 1 shows examples of DMGMI, PMI, and IMAC measurement.
Statistical analysis
The pre-operative and post-operative values were compared using the paired t-test. Pearson’s correlation coefficient (r) between the pre-operative value (pre-DMGMI, pre-PMI, and pre-IMAC) and follow-up change (post-operative value minus pre-operative value defined as ΔDMGMI, ΔPMI, and ΔIMAC) were evaluated. Each sex was separately evaluated. Differences were considered significant when P<0.05. All statistical analyses were performed with commercially available software (Microsoft® Excel and PASW® Statistics 18).
Results
Table 3 shows DMGMI, PMI, and IMAC for pre- and post-LDLT. IMAC was measured for only 11 males and 14 females because 3 males and 3 females did not undergo plain abdominal CT. Figure 2 shows the relationship between pre-DMGMI and ΔDMGMI; r=−0.675 (P=0.008 for males), r=−0.687 (P=0.002 for females). Figure 3 shows the relationship between pre-PMI and ΔPMI; r=−0.739 (P=0.003 for males), r=−0.641 (P=0.006 for females). Figure 4 shows the relationship of pre-IMAC and ΔIMAC; r=0.132 (P=0.700 for males), r=−0.498 (P=0.071 for females). A summary of the results is shown in Table 4.

Full table




Full table
Discussion
The aim of this study was to analyze how trunk muscle mass and adiposity change at 1-year after LDLT in adult patients with liver disease. Our study, which assessed the volume of psoas and dorsal muscle group, demonstrates that sarcopenia is improved after LDLT especially in recipients with smaller muscle mass preoperatively. This tendency was observed both in males and females. On the other hand, those with larger muscle mass at pre-LDLT did not experience apparent muscle volume gain. This may implicate that the subjects in our study included recipients with diverse extent of sarcopenia before LDLT, and those with more severe sarcopenia seem to benefit from LDLT.
In terms of muscle quality assessed by analyzing the adiposity of the dorsal muscle group, significant correlations were not found between before and after LDLT, although muscle adiposity tended to improve in females after LDLT (r=−0.498, P=0.071). This result might suggest that the effect of LDLT on muscle adiposity has sex difference (more benefit in female). Further study with a larger number of patients is need for confirmation. Anyway, sensitivity to change may be greater in muscle mass than muscle adiposity after LDLT for both sexes.
To the best of our knowledge, this is the first study to investigate the effect of LDLT on skeletal muscles according to different muscle mass. Tsien et al. reported that the prevalence of post transplantation sarcopenia is common, and it could not be attributed to pre-transplant characteristics including Child’s score, Model for end-stage liver disease (MELD) score and nutritional status or the type or duration of post-orthotopic liver transplantation (OLT) immunosuppression (17). As our study included only recipients with favorable outcomes, future study with more recipients with severer sarcopenia might clarify the muscle condition required for favorable post-operative outcome.
There are several limitations in this study. First, this is a retrospective study with relatively small number of patients. Second, ROI placement for muscle area and adiposity was evaluated by only one radiological technologist without reproducibility analysis. This was because ROI placement in the muscle was relatively straight-forward and there seemed to be no need to re-test the analysis. However, evaluation of intra- or inter-correlation coefficient may be needed for confirmation, when we consider that measurement with only a slice is likely to cause errors; the imaging slice may change according to the difference of posture and the change in body shape after LDLT. Third, although we measured muscle area rather than muscle volume (18), muscle volume measurement may be more sensitive to change.
In conclusion, improvement of sarcopenia in recipients after LDLT can be demonstrated regardless of sex using volumetric CT.
Acknowledgements
None.
Footnote
Conflicts of interest: The authors have no conflicts of interest declare.
Ethical Statement: This study obtained ethics approval from the ethics committee of Faculty of Health Sciences Hokkaido University (ID16-6). Written informed consent was obtained from the patient for the publication of this manuscript and any accompanying images.
References
- Vieira PM, De-Souza DA, Oliveira LC. Nutritional assessment in hepatic cirrhosis; clinical, anthropometric, biochemical and hematological parameters. Nutr Hosp 2013;28:1615-21. [PubMed]
- Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol 2012;10:117-25. [Crossref] [PubMed]
- Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging 2009;13:724-8. [Crossref] [PubMed]
- Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010;21:543-59. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref]
- Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, Hammad A, Tamai Y, Inagaki N, Uemoto S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014;20:1413-9. [Crossref] [PubMed]
- Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:809-13. [Crossref] [PubMed]
- Ohkawa S, Odamaki M, Yoneyama T, Hibi I, Miyaji K, Kumagai H. Standardized thigh muscle area measured by computed axial tomography as an alternate muscle mass index for nutritional assessment of hemodialysis patients. Am J Clin Nutr 2000;71:485-90. [Crossref] [PubMed]
- Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271-8. [Crossref] [PubMed]
- Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, Harbaugh CM, Holcombe SA, Campbell DA Jr, Sonnenday CJ, Wang SC. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg 2012;256:255-61. [Crossref] [PubMed]
- Glass C, Hipskind P, Tsien C, Malin SK, Kasumov T, Shah SN, Kirwan JP, Dasarathy S. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol (1985) 2013;114:559-65. [Crossref] [PubMed]
- Lee CS, Cron DC, Terjimanian MN, Canvasser LD, Mazurek AA, Vonfoerster E, Tishberg LM, Underwood PW, Chang ET, Wang SC, Sonnenday CJ, Englesbe MJ. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant 2014;28:1092-8. [Crossref] [PubMed]
- Kitajima Y, Eguchi Y, Ishibashi E, Nakashita S, Aoki S, Toda S, Mizuta T, Ozaki I, Ono N, Eguchi T, Arai K, Iwakiri R, Fujimoto K. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol 2010;45:218-24. [Crossref] [PubMed]
- Kitajima Y, Hyogo H, Sumida Y, Eguchi Y, Ono N, Kuwashiro T, Tanaka K, Takahashi H, Mizuta T, Ozaki I, Eguchi T, Kimura Y, Fujimoto K, Anzai K. Japan Nonalcoholic Fatty Liver Disease Study Group (JSG-NAFLD). Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol 2013;28:1507-14. [Crossref] [PubMed]
- Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity (Silver Spring) 2009;17:2082-8. [Crossref] [PubMed]
- Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, Makary M, Hirose K, Edil B, Choti MA, Herman J, Cameron JL, Wolfgang CL, Pawlik TM. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86. [Crossref] [PubMed]
- Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, Fung J, McCullough AJ, Dasarathy S. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol 2014;29:1250-7. [Crossref] [PubMed]
- Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, Kamel IR, Pawlik TM. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 2015;19:1593-602. [Crossref] [PubMed]


