Common and unusual CT and MRI manifestations of pancreatic adenocarcinoma: a pictorial review
Introduction
Pancreatic adenocarcinoma is the most common malignancy of the pancreas with a high mortality, accounting for over 90% of all pancreatic tumors (1). Surgical resection is the only curative treatment option. However, the complication of pancreaticoduodenectomy can be as high as 40% (2,3). Therefore, the accurate characterization of pancreatic adenocarcinoma is very important for patients’ management. CT and MRI have been become the most important modalities for evaluating pancreatic lesions. However, precise diagnosis of pancreatic adenocarcinoma is not always straightforward because they frequently show atypical imaging features and many other diseases may mimic pancreatic adenocarcinoma (4). Understanding its common and uncommon CT and MRI manifestations may facilitate diagnosis and differential diagnosis of pancreatic adenocarcinoma.
CT and MRI protocols
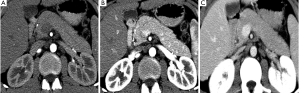
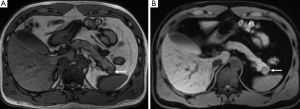
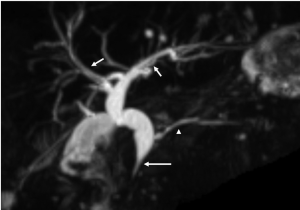
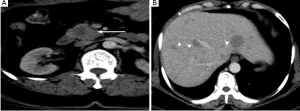
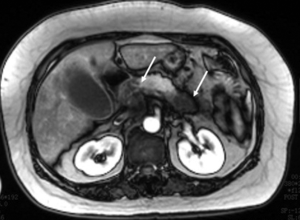
CT and MR imaging protocol have been developed to highlight pancreas. Optimal protocol should obtain obvious contrast between pancreatic parenchyma and lesions. Oral abundant water to fill the gastrointestinal tract is routinely used at CT examination to improve the visualization of peripancreatic anatomic structures. Maximal enhancement can be obtained at pancreatic phase with 40-45 sec delay after intravenous administration of contrast agent at a rate of 3-4 mL/sec. Therefore, radiologists may prefer dual-phase protocol to get optimal enhancement of pancreas and liver due to hypo-vascular characteristics of pancreatic adenocarcinoma. Tri-phase dynamic enhanced CT had been used to characterize pancreas (Figure 1). At arterial dominated phase, most contrast materials are staying in the vascular, the enhancement of the pancreatic parenchyma is mild even though pancreas is a hyper-vascular organ. If neuroendocrine tumor is suspected or clinicians want to know whether arteries are invaded, arterial phase is necessary. Another advantage of pancreatic phase is that peripancreatic vessels, including veins, can be enhanced. This can facilitate assessment of vascular invasion. Conventional MR imaging protocol for pancreas should include T1-weighted images, T2-weighted images, Magnetic resonance cholangiopancreatography (MRCP), and dynamic enhancement with arterial phase, port vein phase, and delay phase using fast gradient recall sequence. Fat-suppress T1 weighted imaging is recommended because it can improve the dynamic contrast of pancreatic parenchyma (Figure 2). MRCP is routinely used in evaluating pancreatic adenocarcinoma to depicture morphological changes of biliary and pancreatic ducts, which has substituted diagnostic ERCP (Figure 3).
Common CT and MRI findings
Direct signs: pancreatic mass
Due to pancreatic duct origin, pancreatic adenocarcinoma often results in pancreatic duct stricture or obstruction, and forms a mass. Approximately 60% occur in the pancreatic head, with the classic clinical presentation of painless jaundice (5).
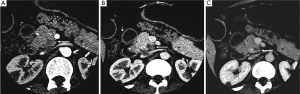
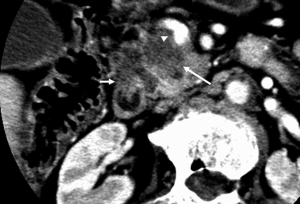
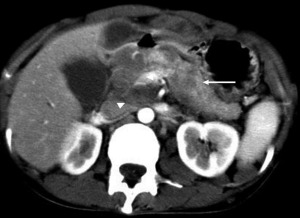
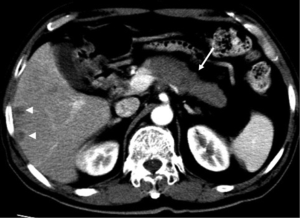
The typical CT and MRI features of pancreatic adenocarcinoma are a hypovascular infiltrative pancreatic mass, often with resultant obstruction of the pancreatic and/or common bile duct (CBD). On CT, it often manifests as a low-density mass on triple phase (arterial and portal) contrast enhanced CT. Maximal contrast between the tumor and the normal pancreas can be achieved in pancreatic and portal venous phase (Figure 4). Because pancreas is a soft organ without capsule and pancreatic adenocarcinoma is extremely invasive, pancreatic adenocarcinoma often had advanced local invasion when it was diagnosed (Figure 5).
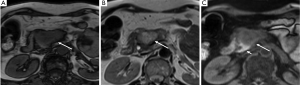
On MR, pancreatic adenocarcinoma shows a mass of hypointensity on T1-weighted image and slight hyperintensity on T2-weighted images, especially on fat-suppressed T1-weighted images (Figure 6). As with CT, dynamic-enhanced MR imaging reveals a low enhancement mass (Figure 7).
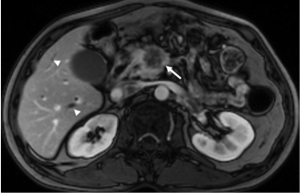
Indirect signs
Pancreatic adenocarcinoma frequently locates at the pancreatic head and cause dilatation of both the pancreatic and common bile duct which is called the “double duct sign” (Figures 8,9). Both CT and MR (esp. MRCP) can show “double duct sign” clearly. Rarely, the tumor in the pancreatic head stricts only CBD, and only the bile ducts are dilatated. When the tumor locates in the neck, body or tail, it causes only upstream pancreatic duct dilatation (Figure 10).
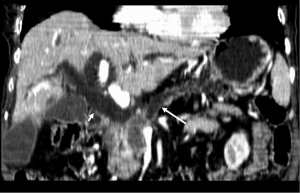
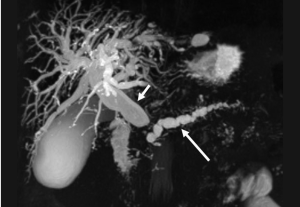
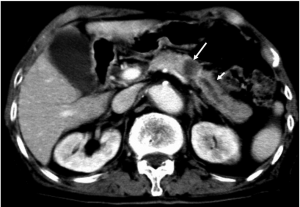
Unresectable disease is seen at presentation in 75% of patients, with metastases (mainly to the liver and peritoneum) presenting in 85% of these patients (6). Tumor invasion of adjacent organs and vessels is also common which makes the tumor unresectable (Figure 11).
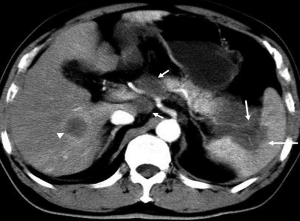
Uncommon radiological findings
Cystic change of pancreatic adenocarcinoma can present, which may be related to a cystic neoplastic component, necrosis, retention cysts from pancreatic ductal obstruction, or pseudocysts from pancreatitis (7) (Figure 12).
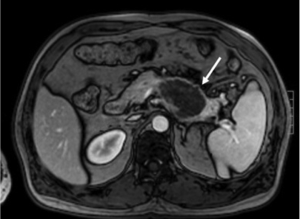
Sometimes, pancreatic adenocarcinoma can present as a focal mass without dilation of upstream pancreatic duct. This phenomenon can be contributed to anatomic variation of pancreatic or bile ducts. If the tumor locates in uncinate process of pancreas, it may not infiltrate bile duct and main pancreatic duct (Figure 13). Multiple lesions might be seen in pancreatic adenocarcinoma rarely (Figure 14). However, it should be differentiated from pancreatic metastasis. The history of primary tumor and the absence of pancreatic duct dilatation can help the differentiation (Figure 15). Occasionally, a pancreatic adenocarcinoma may infiltrate pancreatic parenchyma without distorting pancreatic figuration (Figure 16).
Pancreatic lesions mimics
Since pancreatic adenocarcinoma frequently presents as solid mass in the pancreas, it should be differentiated from solid masses in and adjacent to the pancreas. Common lesions mimic pancreatic adenocarcinoma include: chronic focal pancreatitis, solid pseudopapillary tumors, endocrine tumor of the pancreas, gastrointestinal stromal tumor (GIST) adjacent to the pancreas. Metastatic disease to the pancreas can sometimes be a diagnostic challenge.
(I) Chronic focal pancreatitis can manifest as a focal mass, often in the pancreatic head, thereby mimicking adenocarcinoma (Figure 17). The differential diagnosis between focal pancreatitis and adenocarcinoma may be difficult. Their density and signal intensity can be similar. However, nondilated or smoothly tapering pancreatic and bile ducts coursing through the mass [“duct penetrating sign” (8)], pancreatic duct irregularity, and the presence of pancreatic calcifications favor a diagnosis of focal pancreatitis (9).
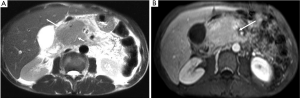
(II) Solid pseudopapillary tumors of the pancreas. This rare cystic low-grade malignancy can sometimes present as a solid mass when it is small making it mimics a small pancreatic adenocarcinoma on unenhanced CT and MR. When scanned at arterial phase, it manifests mild enhancement. However, it has gradual enhancement with time which is not seen in pancreatic adenocarcinoma (Figure 18).
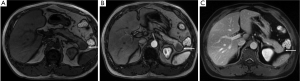
(III) Neuroendocrine tumor of the pancreas frequently presents as a small mass of hypodensity on non-enhanced CT and hypointensity on non-enhanced T1WI. However, it enhances strongly on arterial phase and dilatation of the pancreas duct is usually absent (Figure19).
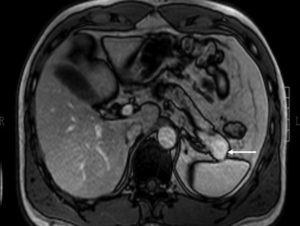
(IV) Sometimes a GIST adjacent to the pancreas can mimic a pancreatic mass (Figure 20). However, GIST has a stronger contrast enhancement than pancreatic adenocarcinoma and rarely has pancreatic duct dilatation.
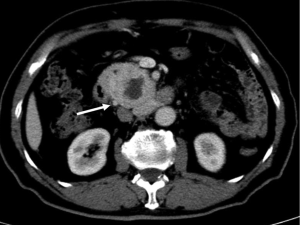
In conclusion, most of pancreatic adenocarcinoma shows typical manifestations on CT and MRI, which can be easy to identify and stage. However, pancreatic adenocarcinoma can demonstrate atypical appearances and several diseases may mimic pancreatic adenocarcinoma on CT and MRI. Familiarity of common and uncommon CT and MRI manifestations of pancreatic adenocarcinoma is essential to achieve the correct diagnose.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mergo PJ, Helmberger TK, Buetow PC, et al. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics 1997;17:281-301.
- Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 2000;232:786-95.
- Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy. Current management. Arch Surg 1992;127:945-9; discussion 949-50.
- Coakley FV, Hanley-Knutson K, Mongan J, et al. Pancreatic imaging mimics: part 1, imaging mimics of pancreatic adenocarcinoma. AJR Am J Roentgenol 2012;199:301-8.
- Martin DR, Semelka RC. MR imaging of pancreatic masses. Magn Reson Imaging Clin N Am 2000;8:787-812.
- Ros PR, Mortelé KJ. Imaging features of pancreatic neoplasms. JBR-BTR 2001;84:239-49.
- Kosmahl M, Pauser U, Anlauf M, et al. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol 2005;18:1157-64.
- Ichikawa T, Sou H, Araki T, et al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology 2001;221:107-16.
- Siddiqi AJ, Miller F. Chronic pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR 2007;28:384-94.


