Aortic stiffness is increased in patients with hypereosinophilic syndrome being in early necrotic phase
Introduction
Persistent eosinophilia and eosinophil-mediated single- or multiple-organ damage are typical features of hypereosinophilic syndrome (HES) (1). Cardiac involvement represents three stages: the early necrotic stage begins with eosinophilic infiltration, followed by an intermediate thrombotic stage and proceeds into a late fibrotic stage (2,3). Transthoracic echocardiography permits measurement of aortic diameter respecting cardiac cycle. Using non-invasively assessed systolic and diastolic blood pressure data, echocardiographic aortic elastic properties could be measured (4). Theoretically, eosinophilic infiltration of the ascending aortic wall could not be excluded in HES, therefore the present study aimed to test whether HES in early necrotic phase is associated with abnormalities in aortic elastic properties.
Methods
Patient population
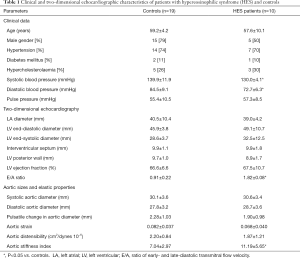
The present study comprised 10 HES patients (mean age: 57.6±10.1 years, 5 males) in the earliest presumably early necrotic phase. Available guidelines were used to confirm the diagnosis of HES in all patients (5). Their results were compared to 19 age-, gender- and risk factor-matched controls (mean age: 59.2±4.2 years, 15 males). None of HES patients and controls had cardiovascular events in their medical history including thrombosis and all were asymptomatic at the time of examination. The following non-cardiovascular organ involvements were found in this HES patient group: duodenal eosinophilia in 1 case, tissue (pulmonary) eosinophilia in 1 case, eosinophilic dermatitis in 1 case, sensory-motor neuropathy with pulmonary involvement and granulomatous necrotizing vasculitis confirmed with sural biopsy in 1 case and sole skin involvement in 1 case. Clinical data and risk factors of each group are presented in Table 1. None of hypereosinophilic patients or controls had chronic obstructive pulmonary disease, history of pulmonary embolism, atrial septal defect or other malignancies. The laboratory findings proved to be the followings in HES patients: serum red blood cell level: 4.08±0.40 T/L, haemoglobin level: 130.0±16.0 g/L, platelet level: 267.2±177.0 Giga/L, haematocrit: 37.8%±4.5%, white blood cell level: 14.4±6.8 Giga/L, ratio of eosinophils: 47.6%±18.1% and absolute number of eosinophils: 8.1±5.3 Giga/L. The protocol was conformed to the ethical guidelines of the 1975 Declaration of Helsinki, the institutional review board approved it and each subject gave informed consent.
Full table
Biochemical measurements
Blood samples were withdrawn by venipuncture after 8 hours of overnight fasting to evaluate routine blood parameters.
Blood pressure measurement
After recording demographic and clinical data, systolic and diastolic blood pressures (SBP and DBP, respectively) were measured in the supine position with a mercury cuff sphygmomanometer from left arm after 10 min of rest. The first and the fifth Korotkoff sounds were used to identificate SBP and DBP. Stimulant consumption was not allowed from 30 minutes before the blood pressure measurements. The average of three consecutive measurements corresponded to blood pressure measurement.
Two-dimensional Doppler echocardiography
Transthoracic imaging was performed by experienced investigators with a 1–5 MHz PST-30SBP phased-array transducer using a Toshiba ArtidaTM cardiac ultrasound system (Toshiba Medical Systems, Tokyo, Japan). Complete 2D Doppler and tissue Doppler echocardiographic study were undertaken following recent guidelines with the patient in the left lateral decubitus position from multiple windows. All echocardiographic studies were digitally stored and measurements were averaged from three beats. Echocardiographic dimensions and ejection fraction were measured regarding to the recent guidelines (6). Colour Doppler echocardiography was used to visually quantify degree of valvular regurgitations and pulsed Doppler to perform mitral inflow E/A measurements.
Assessment of echocardiographic aortic elastic properties
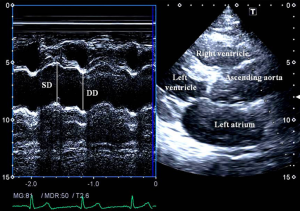
Aortic elastic properties were calculated according to the literature (Figure 1) (7). To evaluate aortic properties systolic and diastolic ascending aortic diameters (SD and DD) were measured at the end of systole and diastole by M-mode echocardiography at a level of 3 cm above the aortic valve in a parasternal long-axis image. The SD and DD were assessed at the time out of maximum aortic anterior motion and at the peak of the QRS complex on the recorded electrocardiogram, respectively. The following aortic elasticity parameters have been calculated:
- Aortic strain = (SD – DD)/DD;
- Aortic stiffness index (β) = ln (SBP/DBP)/[(SD − DD)/DD], where SBP and DBP are the systolic and diastolic blood pressures, and ‘ln’ is the natural logarithm;
- Aortic distensibility = 2× (SD – DD)/[(SBP – DBP) × DD].
Statistical analysis
Variables are given as mean ± standard deviation or number (percentage) of patients. All statistical tests were 2-sided and statistical significance was established at a level of <0.05. Categorical variables were examined by chi-square test and Fisher’s exact test, while continuous variables were measured with the unpaired Student t-test. Numerical correlations were determined by Pearson’s correlation. MedCalc software (MedCalc, Inc., Mariakerke, Belgium) was used for statistical analyses.
Results
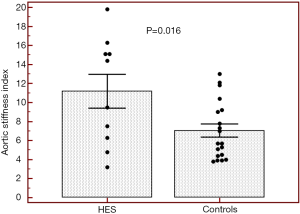
Routine two-dimensional echocardiographic data with aortic measurements are presented in Table 1. None of controls and HES patients showed > grade 1 mitral or tricuspid regurgitations. Although neither systolic nor diastolic aortic diameters differed significantly between HES patients and matched controls, significantly increased aortic stiffness index could be demonstrated suggesting vascular remodeling in HES patients (Table 1, Figure 2). No significant correlations could be demonstrated between echocardiographic aortic elastic properties and laboratory findings.
Discussion
Increased arterial stiffness is one of the earliest indicators of vascular dysfunction, an important cardiovascular risk factor and a predictor of clinical outcomes (8,9). Using echocardiographically assessed aortic diameter data respecting cardiac cycle in combination with forearm blood pressure values, a number of echocardiographic aortic elasticity parameters could be calculated (4,7). Distensibility of the ascending aorta determined such a non-invasive way was found to be closely related to that obtained by direct invasive measurements (4).
Cardiac manifestations follow three stages in HES: the first acute, mostly asymptomatic necrotic stage is due to myocardial infiltration of eosinophils (2,3,10). Thrombus formation followed by its organization into a thick layer of granulation tissue could be demonstrated in the thrombotic phase. In the last fibrotic stage granulation tissue changes into fibrosis. Endocardial fibrous thickening, LV apical fibrothrombotic obliterations and valvular regurgitations are typical echocardiographic findings in HES (2,3,10).
During youth, the aorta is relatively elastic, but is known to stiffen with age. Classic cardiovascular risk factors play a significant role in accelerating this process (11). Aortic stiffness is associated with coronary artery disease, hypertension, diabetes mellitus, obesity, end-stage renal disease, older age, etc., and is an independent predictor of vascular morbidity and mortality (8,11-13). Aortic wall fibrosis, medial smooth muscle cell necrosis, presence of breaks in elastic fibers, calcifications, and macromolecule diffusion into the arterial wall are known features in these conditions leading to increased aortic stiffness (7,8). In the present study increased aortic stiffness could be demonstrated in HES patients being in early necrotic phase without obvious cardiovascular alterations or events in their medical history as compared to matched controls. These results could be theoretically explained by eosinophil infiltration of the aortic wall and related tissue damage. However, the effects of asymptomatic myocardial infiltration and associated pumping dysfunction, and other risk factors could not be excluded.
There are limitation sections with this study. Only ten HES patients were involved into the present study which limited statistical analyses. However, HES is a relatively rare disease. As mentioned above, some HES patients had cardiovascular risk factors which could theoretically affect results. Coronary angiography was not performed in any of our cases or controls to exclude coronary artery disease. Although control subjects had higher blood pressure values at the time of measurement, their aortic elasticity parameters proved to be better as compared to that of HES patients. These facts strengthen our findings since aortic stiffness index was greater in HES patients although their blood pressure was better controlled.
In conclusions, this study suggests that alterations in echocardiographic aortic elastic properties could be demonstrated in HES patients being in early necrotic phase.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was conformed to the ethical guidelines of the 1975 Declaration of Helsinki, the institutional review board approved it and each subject gave informed consent.
References
- Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood 1994;83:2759-79. [PubMed]
- Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart 2016;102:100-6. [Crossref] [PubMed]
- Kleinfeldt T, Nienaber CA, Kische S, Akin I, Turan RG, Körber T, Schneider H, Ince H. Cardiac manifestation of the hypereosinophilic syndrome: new insights. Clin Res Cardiol 2010;99:419-27. [Crossref] [PubMed]
- Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J 1990;11:990-6. [Crossref] [PubMed]
- Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol 2014;89:325-37. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Nemes A, Geleijnse ML, Forster T, Soliman OI, Ten Cate FJ, Csanády M. Echocardiographic evaluation and clinical implications of aortic stiffness and coronary flow reserve and their relation. Clin Cardiol 2008;31:304-9. [Crossref] [PubMed]
- Belz GG. Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther 1995;9:73-83. [Crossref] [PubMed]
- Nemes A, Forster T. Evaluation of vascular function by modern non-invasive methods. Orv Hetil 2012;153:1887-95. [Crossref] [PubMed]
- ten Oever J, Theunissen LJ, Tick LW, Verbunt RJ. Cardiac involvement in hypereosinophilic syndrome. Neth J Med 2011;69:240-4. [PubMed]
- Sethi S, Rivera O, Oliveros R, Chilton R. Aortic stiffness: pathophysiology, clinical implications, and approach to treatment. Integr Blood Press Control 2014;7:29-34. [Crossref] [PubMed]
- Bader H. Importance of the gerontology of elastic arteries in the development of essential hypertension. Clin Physiol Biochem 1983;1:36-56. [PubMed]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318-27. [Crossref] [PubMed]


