Gastrointestinal tract involvement in acute pancreatitis: initial findings and follow-up by magnetic resonance imaging
Introduction
Acute pancreatitis (AP) is a common clinical problem. The median mortality of AP is 10% but may reach 20–30% in severe acute pancreatitis (SAP) with multiple organ dysfunction syndromes (1). The most commonly involved organ in AP and particularly in SAP is the gastrointestinal tract, which is correlated with poor outcomes in AP patients (2). Assessing the severity of AP and gastrointestinal tract involvement for the proper management of AP patients are critical components of any strategy designed to reduce AP mortality (3).
Magnetic resonance imaging (MRI) has an excellent resolution of soft tissue, and it has evolved as a valuable tool to non-invasively identify AP complications, trouble shoot difficult cases of AP and assess response to treatment without the radiation exposure. MRI can accurately evaluate the necrosis, hemorrhage, ductal system and inflammation associated with AP (4-6). Compared with traditional examination methods, such as endoscopy, barium meals and CT scans, MRI has some unique advantages, including improved soft tissue contrast, and no ionizing radiation (7). Therefore, the use of MRI to evaluate the gastrointestinal tract is gradually increasing (7-10).
In clinical studies of intestinal complications of AP, González Jiménez et al. (11) described five SAP patients who had duodenal stenosis. Lal et al. (12) reported a case of large bowel obstruction in a 31-year-old postpartum female, secondary to severe gallstone pancreatitis, Liu et al. (2) reported that intestinal mucosal function was injured in early phase of AP especially in patients with organ dysfunction, which may be a stimulus for development of multiple organ dysfunction and correlate with bad outcome in AP patients. Animal experiments (13) revealed that gastric emptying and intestinal peristalsis were decreased by acute necrotizing pancreatitis and undiagnosed gastrointestinal tract damage may lead to fatal outcome if an enterogenic infection occurs. Chen et al. (14) found that 65% of patients of AP complicate with acute gastrointestinal mucosal lesions. In the imaging studies, as early as in 1980, Mendez et al. (15) reported that 5 of 102 AP patients had involvement of the transverse mesocolon and small bowel mesentery in CT examinations. Liu et al. (16) observed that gas volume was significantly elevated in patients with AP in plain abdominal radiographs. Raghuwanshi et al. (17) found that 88% AP patients had mesentery, greater omentum and transverse mesocolon abnormalities on CT examination. However, these examinations are not very sensitive and are generally inadequate to demonstrate the full extent of the gastrointestinal tract abnormalities associated with AP and the minute structure changes of gastrointestinal tract could be clearly demonstrated with MRI. Furthermore, MRI doesn’t involve radiation, it can be repeatedly used for follow-up.
We conducted the current study to assess gastrointestinal tract involvement in AP with MRI and analyzed the severity of gastrointestinal tract abnormalities on MRI in relation to those abnormalities evaluated by the MR severity index (MRSI) and the Acute Physiology and Chronic Healthy Evaluation II (APACHE II) scoring system. MR follow up of the gastrointestinal tract was used to evaluate the therapy effect of AP.
Methods
Patient population
Our institutional review board approved this retrospective study and informed consent was waived since this was a retrospective study. The inclusion criteria for the study of AP were: (I) acute history; (II) pancreatitis at first onset; (III) three-fold elevated amylase or lipase, excluding other causes of elevated enzymes; and (IV) abdominal MR examinations within 2 days of the pancreatitis onset. The exclusion criteria were: (I) inability to cooperate when performing MRI; (II) incomplete clinical records; (III) history of chronic pancreatitis; (IV) AP due to pancreatic carcinoma; (V) history of gastrointestinal surgery; and (VI) digestive tract diseases, such as neoplastic lesions or inflammatory disease, and other diseases which could lead to gastrointestinal tract abnormalities (18,19).
Two hundred and thirty-three patients with AP were admitted to our institution between October 2015 and April 2017. Twenty-four patients were excluded, including five patients with severe motion artifacts during MRI scanning, five patients with appendix operations, two patients with esophageal cancer, five patients with gastric cancer, three patients with colorectal cancer, one patient with pancreatic cancer, and three patients with cirrhosis. The final study group consisted of 209 consecutive AP patients, 62 of whom had follow-up MRI.
The control group was selected from the patients in our daily clinical caseload who underwent MRI over the same recruitment period. The inclusion criteria for the control group were as follows: (I) adequate bowel wall visualization; (II) nonspecific abdominal symptoms, such as nausea, vomiting, abdominal pain with diarrhea, bloody diarrhoea; (III) had no other disease which can cause abnormality of gastrointestinal tract, such as cirrhosis, portal hypertensive, ascites and heart failure. The exclusion criteria for control group were as follows: (I) AP; (II) history of neoplasms of digestive system; (III) history of intraperitoneal tumor; (IV) had previous abdominal surgical interventions; (V) other digestive tract diseases, such as inflammatory disease and ileus.
MRI techniques
MRI was performed on a 1.5-T MR scanner with 38 mT/M gradients and 120 mT/M per second slope (Signa Excite; GE Medical Systems, Milwaukee, WI, USA) using a phased array torso-pelvis coil. No antiperistaltic agent or enteric contrast was administered. The coverage area of axial scan was apex of the liver to the umbilical. The sequences included axial fast spoiled gradient echo (FSPGR) T1-weighted imaging with fat suppression [repetition time (TR) ms/echo time (TE) ms =150–170/1.6; flip angle =80°; matrix =512×160–192; field of view (FOV) =26–32 cm; section thickness =5 mm; number of excitation (NEX) =1], gradient-echo (GRE) T1-weighted in-phase and out-of-phase MR imaging (TR ms/TE ms =150/4.4, 2.2; flip angle =90°; matrix =256×192–224; FOV=26–32 cm; section thickness =5 mm; NEX =1), respiratory-triggered (R-T) axial fast recovery fast spin-echo (FRFSE) T2-weighted MR imaging with fat suppression (TR ms/TE ms =10,000–12,000/90–100, TR determined by the frequency of respiration; section thickness = 5 mm; intersection gap =0.5 mm; matrix =256×192; NEX =3; and FOV =34 cm × 34 cm), coronal and axial single shot fast spin-echo (SSFSE) T2-weighted MR imaging (TE =90–100 ms; 2 s between slice acquisitions; section thickness =5 mm; intersection gap =0.5 mm; matrix =384×224; one-half signal acquired; and FOV =33 cm × 33 cm), SSFSE radial series slabs MR cholangiopancreatography (MRCP) (TE =1,300 ms; 6 s between image acquisitions; section thickness =40 mm; matrix =384×224; one-half signal acquired; FOV =30 cm × 30 cm), and three-dimension (3D) FSPGR dynamic enhanced MR imaging. Dynamic enhanced imaging was performed with an axial fat saturated 3D FSPGR sequence. Gadolinium chelate (Magnevist, Schering Guangzhou Co., China) was administered (0.2 mmol/L per kilogram of body weight) intravenously at approximately 3.5 mL/s by projector (Spectris MR Injection System, Medrad Inc., USA) injection, followed by a 20-mL saline solution flush. First-pass arterial enhancement was optimized with a timing bolus sequence (axial FMPSPGR). Dynamic imaging was performed during breath-holding before the injection (unenhanced), immediately after the injection (hepatic arterial phase), 30 s after the injection (early venous phase), and 1 min after the injection (late venous phase). An additional delayed phase was acquired using 2D FSPGR fat suppression axial T1-weighted imaging.
MRI interpretation
The original MRI data were transferred to a workstation (GE, AW4.1, Sun Microsystems, Palo Alto, CA, USA) to be reviewed. Two observers (with 5 and 8 years of experience in interpreting abdominal MRI, respectively) blinded to the laboratory data and clinical outcomes independently reviewed pancreas on the MRI. Another two observers (with 5 and 8 years of experience in interpreting abdominal MRI, respectively) blinded to the laboratory data and clinical outcomes independently reviewed the gastrointestinal tract.
On the MR images, AP was categorized as edematous and necrotic pancreatitis (20). The severity of AP was graded according to MRSI (20). According to MRSI, AP was divided into mild, moderate and severe APs (MRSI was 0–3, 4–6, 7–10 points separately) (20,21). We divided the gastrointestinal tract anatomy into stomach, duodenum, jejunum, ileum, ascending colon (including the ileocecal junction), transverse colon (including the hepatic flexure and splenic flexure), descending colon and the sigmoid colon. Wall thickening was defined as over 10 mm for stomach wall and over 3 mm for intestine, when their lumens were well distended (22-24). Abnormalities of the thickened gastrointestinal tract walls included the degree of thickening, signal intensity change, and symmetric versus asymmetric thickening. Lumen dilatation was defined as diameter over 30 mm for small intestine and over 60 mm for the colon (24).
The change in the bowel wall signal intensity was compared with adjacent normal bowel on T2WI and contrast enhanced T1WI. When the bowel wall signal intensity was close to that of the normal bowel, we defined it as isotense. When the signal intensity was much higher than that of normal bowel wall, it was defined as hyperintense, and when it was much lower than normal bowel wall, it was defined as hypointense.
Although the axial scans did not include lower abdomen, the entire gastrointestinal tract was evaluated on the coronal and sagittal scans.
APACHE II score
In all 209 patients, the APACHE II score was calculated using 13 common physiological and laboratory values from the first 48 hours after admission without knowledge of the MRI findings, obtained from the medical records by clinician or nurse. According to the standard of Atlanta, an APACHE II score of 8 was selected as a cut-off point to differentiate between mild and severe cases (25,26).
The medical records of AP patients were reviewed for observing the recovery of gastrointestinal functions, including relief of abdominal pain, recovery of eating, hematuria, amylase recovery and anal passing gas.
Statistical analysis
All of the quantitative data derived from MRI findings are reported as the average of the two observers and qualitative data were negotiated by the two raters. Kappa statistics were used to assess the inter-rater reliability between the two reviewers.
The MRSI and APACHE II scores are reported as ranges and as the mean ± standard deviation. The frequencies of gastrointestinal tract abnormalities were calculated in the AP and in the control groups, and the Chi-squared test or Fisher exact test was applied to determine the significance of their difference. Chi-squared test or Fisher exact test was used for the differences in the prevalence of gastrointestinal tract abnormities in mild, moderate, and severe AP according to the MRSI scores or as mild or severe AP according to the APACHE II scores. The Spearman’s rank correlation coefficient was calculated to test the correlation of the gastrointestinal tract abnormality with the MRSI and the APACHE II scores. Initial and follow up MRI examinations of gastrointestinal tract abnormalities were compared by using the Chi Square Test or Fisher exact test and paired sample t-test.
All statistical tests were calculated using Statistical Package for Social Sciences (SPSS) for Windows (Version 11.5, Chicago, IL, USA). P<0.05 was considered significant.
Results
Patient sample
Of the 209 patients with AP, there were 107 men and 102 women with an average age of 53±16 years (range, 16–82 years). The etiology for AP was biliary in 50% (105/209), high-fat diet in 18% (37/209), alcoholic in 5% (10/209), postoperative status in 1% (3/209), and undetermined in 26% (54/209) of patients. Of the 62 patients with follow up MRI examination, there were 40 men and 22 women with an average age of 53±14 years (range, 22–80 years). The etiology for AP was biliary in 52% (32/62), high-fat diet in 16% (10/62), alcoholic in 13% (8/62), and undetermined in 19% (12/62) of patients. All patients accept fasting until they can tolerate an oral diet.
In the control group, there were 53 men and 47 women with an average age of 46±12 years (range, 13–71 years). Thirty-seven patients were normal on MRI, twenty-four patients had renal cysts, eighteen patients had liver cysts, eleven patients had liver hemangiomas, and ten patients had splenomegaly.
MRI finding of AP
Of the 209 patients with AP, 33% (68/209) received dynamic enhanced MRI. Sixty-two patients had followed up MRI examination in 13±8 days (range, 3–49 days) after admission. The agreement between the observers for MRSI was satisfactory (κ=0.73, P<0.05). Approximately 35%, 59% and 6% of the patients had mild, moderate and severe APs, respectively according to MRSI. The MRSI score averaged 3.1±1.8 points (range, 0 to 10 points).
Approximately 63% (132/209) of the patients with AP had at least one gastrointestinal tract abnormality in the initial MRI examination, compared with 5% (5/100) of the patients in the control group (P<0.05). Among control subject, the gastrointestinal tract abnormalities included smooth mild stomach wall thickening with no mural stratification (r=2), smooth mild duodenum wall thickening with no mural stratification (r=1), dilatation of the gastrointestinal tract (r=2). The agreement of the two raters was generally good (Table 1).
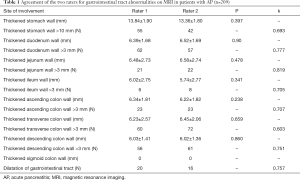
Full table
The prevalence of gastrointestinal tract abnormalities was 47% in mild AP, 93% in moderate AP and 100% in severe AP, respectively (among the three groups, P<0.05). Gastrointestinal tract abnormalities were correlated with the MRSI score (r=0.46, P<0.05).
Gastrointestinal tract abnormalities on MRI were noted in 132 patients, including mild smooth gastrointestinal tract wall thickening, mural stratification, and bowel dilatation. Thickened bowel wall was noted in 45 patients, which showed diffuse enhancement in 22 patients and stratified enhancement in 23 patients during the arterial phase after Gd intravenous injection.
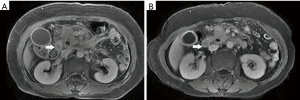
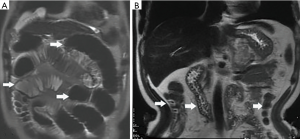
Among the AP patients, wall thickening involved duodenum (Figure 1) (27%), ascending colon (26%), stomach (20%), transverse colon (15%), jejunum (Figure 2) (14%) and ileum (6%). Dilatation of the gastrointestinal tract was seen in 8% (Figure 2, Table 2).
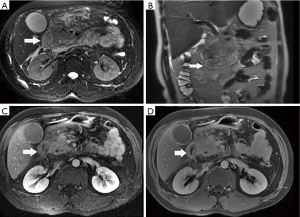
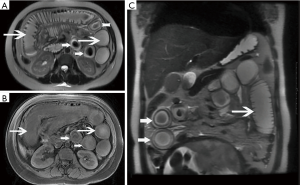
Full table
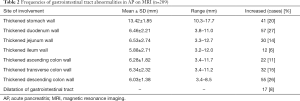
During treatment, 62 patients had follow up MRI examination, 16 of whom (26%) had a gastrointestinal tract abnormality on follow up examination, significantly lower than that on the initial MRI (P<0.05) (Figures 3-7). The frequencies of gastrointestinal tract abnormalities for initial and follow-up MRI examination are shown in Table 3. The difference for the frequencies of stomach, duodenum, jejunum, ileum, ascending and descending abnormal changes between before and after the treatment had statistical significance (P<0.05). There was no difference of transverse colon and bowel dilatation before and after the treatment (P≥0.05).




Full table
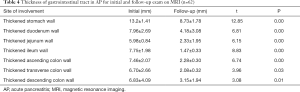
It was found that gastrointestinal tract wall thickness was greatly thinner after treatment (P<0.05) (Table 4).
Full table
The APACHE II score
Among the 209 patients with AP, the mean APACHE II score was 5.98±4.0 points (ranging from 0 to 23 points). A total of 150 patients had mild AP (APACHE II score <8 points), whereas 59 patients had severe AP (APACHE II score ≥8 points). Approximately 83% (49/59) of the patients with severe AP had gastrointestinal tract abnormalities, significantly more than the 57% (86/150) of patients with mild AP and gastrointestinal tract abnormalities (P<0.05). The presence of gastrointestinal tract abnormalities was slightly correlated with the APACHE II score (r=0.19, P<0.05).
In the follow-up MRI exam, the structure of gastrointestinal tract restored to revert in MRI, coinciding with relief of abdominal pain, recovered appetite, cessation of hematuria, amylase recovery and anal passing gas.
Discussion
In this study, 63% of patients had at least one gastrointestinal tract abnormality on MR imaging. The patterns of the abnormalities included even and mild gastrointestinal tract wall incrassate, mural stratification, stratification enhancement on artery phase after Gd intravenous injection, and dilatation of gastrointestinal tract. The most common abnormalities included thickened duodenal wall (27%), thickened descending colon wall (26%), and thickened stomach wall (20%). The prevalence of gastrointestinal tract abnormalities was 47% in mild AP, 93% in moderate AP and 100% in severe AP, respectively. Gastrointestinal tract abnormalities were correlated with the MRSI score. It was found that gastrointestinal tract wall thickness was greatly thinner after treatment. Our results depicted the patterns and the evolution of gastrointestinal tract in AP by using MRI which may be helpful for early recognition and institution of appropriate therapy to improve the prognosis of AP. We found the prevalence of gastrointestinal tract abnormality was 63% which was higher than previous studies, in which gastrointestinal tract abnormality was exceedingly rare, and only isolated case reports had described them (11,12). Our results demonstrate that the prevalence of gastrointestinal tract abnormality is higher in MRI study than previous trials. The possible reason may be that MRI is more sensitive to depict the slight changes in gastrointestinal tract in AP.
In this study gastrointestinal tract wall thickening was the most common finding in AP patients. Inflammatory substances can stimulate the gastrointestinal tract in patients with AP, leading to the excessive output of gastric juices and subsequent mucosal and submucosal edema (27). Some patients exhibit a three-layer structure referred to as the “target sign” (28). Tolan et al. (29) considered this pattern acute wall edema, and noted a direct correlation with inflammatory activity. In this study we found most AP patients exhibited gastrointestinal tract wall thickening, some with a stratified appearance on MRI that was similar to the “target sign” (28). After treatment, the mural stratification resolved on follow-up MRI. Our results suggested that the thickened intestinal wall in AP patients is derived from acute wall edema and can resolve soon after treatment.
We found frequent mild smooth thickening of the intestinal wall, similar to that of other non-tumorous bowel wall thickening patterns. Ramalho et al. (30) observed that the wall thickness usually ranges between 5 and 10 mm for patients with small bowel Crohn’s disease, similar to the range we noted (Table 2) in AP patients. Bowel wall thickening has been observed in bowl ischemia edema, bleeding and infection, although this thickness was unrelated to disease severity (31). Gastrointestinal tract wall thickness reduced after treatment, suggesting resolved edema after effective treatment.
We noted two patterns of wall enhancement in the initial exam; diffuse, and stratified. Tolan et al. (29) proposed that the patterns of wall enhancement could help determine the likely level of disease activity. Tolan et al. reported that stratified enhancement was observed in combination with submucosal edema in active disease and that diffuse enhancement commonly represented transmural inflammation. Low-level inhomogeneous enhancement was often observed in fibrosis (22). We consider that the wall thickening we observed with AP resulted from active inflammation, and that this inflammation was transmural in some cases. In the follow-up examination, we noted smooth mild wall thickening with no mural stratification, confirming that active inflammation could resolve soon after treatment.
AP inflammation can spread through connected anatomic compartments, or across biofilms damaged by pancreatic enzymes (32,33). The closer to the pancreas, the more obvious the impact on the affected tissues and organs. As duodenum, ascending colon and descending colon are retroperitoneal and stomach, jejunum and transverse colon are close to the pancreas (34), we found these organs could be frequently involved in this study. In contrast, the sigmoid colon, far from the pancreas, showed no involvement in this study.
The pattern of wall thickening is not sufficient to identify the cause of thickened bowel wall, although a characteristic distribution of involvement may help identify specific diseases. For example, thickening of the ascending colon and jejunum are common in cirrhosis (18). Intestinal mechanical obstruction usually occurs in the small intestine (35). Crohn’s disease usually localizes to the distal ileum (29). The most commonly affected intestine in ulcerative colitis is the left colon (9). Mesenteric vascular lesions are associated with ischemic bowel disease (36). Immune responses and hypoalbuminemia often affect both the small and large intestines (37). The most commonly involved segment is the large intestine in inflammatory enteritis (38). In blunt abdominal trauma, bowel injury in included bowel wall transaction discontinuity, extraluminal air, focal bowel wall thickening, free peritoneal fluid, mesenteric infiltration or hematoma on CT examination. Duodenal was most commonly affected bowel in pancreatic injuries (39). In this study we found the most commonly affected intestines in AP were duodenum and descending colon. One thing we need to pay special attention to is AP patients with inflammatory bowel disease (IBD). Antonini et al. (40) found that patients with IBD have increased risk of AP. In this study, no AP patients had IBD. AP and IBD may have similar presentation, therefore a pancreatic disease could not be recognized in patients with IBD. However, elevation of pancreatic enzymes only, without symptoms nor imaging suggestive of pancreatitis, is not an indication to start a treatment but is recommended to follow up in first instance. On the other hand, patients with IBD presenting with pancreatitis-like abdominal pain should be investigated to rule out a concomitant pancreatic disease. Liu et al. (16) observed that gas volume of gastrointestinal in was significantly elevated in patients with AP. In this study, we also found some AP patients had dilatation of the gastrointestinal tract with gas. But it was a nonspecific finding and can occur in various diseases (41).
In this study, no patient had gastrointestinal fistula or bowel obstruction which is rare complication of SAP but one that clinicians should be aware of due to its high mortality. According to the previous research, the sites of fistula may involve any site of gastrointestinal, either in localization or diffusion (42-44). Most of the cases were confirmed clinically 4–8 weeks after onset of the disease, which suggests that the development of gastrointestinal fistula is associated with the long-term effects of the pancreatic or peripancreatic inflammation and infection (44). In our study, the finding is in disagreement with the results, none of AP patients has gastrointestinal fistula or obstruction. In our study, AP patients had a small number of SAP patients (6%) and rather short-term follow up (62 patients had follow up MRI examination in 13±8 days after admission). As we found in our study, colonic and duodenal were the most easily involved structures. Colonic and duodenal fistulas are the two commonest forms of fistula in SAP. For the clinical outcome, the occurrence of colonic fistula and obstruction is associated with worse outcome (45).
Optimal feeding has always been regarded as an important means to improve the outcome in AP patients. Starting early enteral feeding as soon as possible was thought to be important, but until recently, no scientific study was available to determine the exact time and it remains debatable (46,47). In clinical therapy, if patients can tolerate an oral diet without abdominal pain, nausea, vomiting, or ileus, they may be allowed to have oral food (48). In this study, all patients accept fasting until they can tolerate an oral diet, we found the gastrointestinal tract abnormalities resolve when their abdominal symptoms improved significantly. Gastrointestinal tract monitoring with MRI may provide objective index for the recovery of bowel function, exact time of enteral feeding and curative effect.
MRSI exhibits advantages in evaluating local complications (49). APACHE II scores are the “traditional” multifactorial scoring systems for predicting the severity, pancreatic necrosis, and mortality of AP (50). In this study we found that gastrointestinal tract abnormalities on MRI increased with the increasing severity of AP. Gastrointestinal tract abnormalities were correlated with the MRSI and APACHE II scores. We speculate that gastrointestinal tract abnormalities were mainly due to massive pancreatic fluid leakage.
There are several limitations of this study. Firstly, some patients with AP were treated by conservative therapy including fasting and nasogastric drainage, and gastrointestinal preparation cannot be made before MRI examination. This has a great influence on observing the intestinal wall. In order to increase the accuracy of the study, scans from a control group of patients without AP were included in this study. Secondly, the time interval between MRI examination and the onset of AP is variable, which may have affected the prevalence of gastrointestinal tract and the MRSI scores. Therefore, we performed MRI within 48 h after admission to minimize variability.
In conclusion, gastrointestinal tract plays an important role in the development of AP. Early recognition and institution of appropriate therapy may improve prognosis of AP. Gastrointestinal tract abnormalities on MRI are common in AP and they are positively correlated with the severity of AP on MRI and may add value for determining the severity of AP. MRI can be used to monitor treatment effects of gastrointestinal tract abnormity and can provide objective index for the recovery of bowel function, exact time of enteral feeding and curative effect.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our institutional review board approved this retrospective study and informed consent was waived since this was a retrospective study.
References
- Landahl P, Ansari D, Andersson R. Severe Acute Pancreatitis: Gut Barrier Failure, Systemic Inflammatory Response, Acute Lung Injury, and the Role of the Mesenteric Lymph. Surg Infect (Larchmt) 2015;16:651-6. [Crossref] [PubMed]
- Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 2008;36:192-6. [Crossref] [PubMed]
- Koh YY, Jeon WK, Cho YK, Kim HJ, Chung WG, Chon CU, Oh TY, Shin JH. The effect of intestinal permeability and endotoxemia on the prognosis of acute pancreatitis. Gut Liver 2012;6:505-11. [Crossref] [PubMed]
- Chi XX, Chen TW, Huang XH, Yang L, Tang W, Wáng YX, Xiao B, Zhang XM. Magnetic resonance imaging of retroperitoneal interfascial plane involvement in acute pancreatitis. Quant Imaging Med Surg 2016;6:250-8. [Crossref] [PubMed]
- Tang MY, Chen TW, Huang XH, Li XH, Wang SY, Liu N, Zhang XM. Acute pancreatitis with gradient echo T2*-weighted magnetic resonance imaging. Quant Imaging Med Surg 2016;6:157-67. [Crossref] [PubMed]
- Liu N, Huang XH, Zhang XM, Dong GL, Jing ZL, Gao CL, Tang MY. The angle of pancreaticobiliary junction correlates with acute pancreatitis: a magnetic resonance cholangiopancreatography study. Quant Imaging Med Surg 2015;5:401-6. [PubMed]
- Markova I, Kluchova K, Zboril R, Mashlan M, Herman M. Small bowel imaging - still a radiologic approach? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010;154:123-32. [Crossref] [PubMed]
- de Zwart IM, de Roos A. MRI for the evaluation of gastric physiology. Eur Radiol 2010;20:2609-16. [Crossref] [PubMed]
- Gee MS, Harisinghani MG. MRI in patients with inflammatory bowel disease. J Magn Reson Imaging 2011;33:527-34. [Crossref] [PubMed]
- Amitai MM, Raviv-Zilka L, Hertz M, Erlich Z, Konen E, Ben-Horin S, Apter S. Main Imaging Features of Crohn's Disease: Agreement between MR-Enterography and CT-Enterography. Isr Med Assoc J 2015;17:293-7. [PubMed]
- González Jiménez MA, Calvete Chornet J, Sanahuja Santafé A, García-Granero Ximénez E, Martín Espinosa F, Lledó Matoses S. Duodenal stenosis: a rare complication of acute pancreatitis. Rev Esp Enferm Dig 1997;89:565-8. [PubMed]
- Lal N, Whiting J, Hejmadi R, Raman S. Large Bowel Obstruction, a Delayed Complication of Severe Gallstone Pancreatitis. Case Rep Surg 2016;2016:1034929. [PubMed]
- Seerden TC, De Winter BY, Van Den Bossche RM, Herman AG, Pelckmans PA, De Man JG. Regional differences in gastrointestinal motility disturbances during acute necrotizing pancreatitis. Neurogastroenterol Motil 2005;17:671-9. [Crossref] [PubMed]
- Chen TA, Lo GH, Lin CK, Lai KH, Wong HY, Yu HC, Hsu PI, Chen HH, Tsai WL, Chen WC. Acute pancreatitis-associated acute gastrointestinal mucosal lesions: incidence, characteristics, and clinical significance. J Clin Gastroenterol 2007;41:630-4. [Crossref] [PubMed]
- Mendez G Jr, Isikoff MB, Hill MC. CT of acute pancreatitis: interim assessment. AJR Am J Roentgenol 1980;135:463-9. [Crossref] [PubMed]
- Liu Y, Luo HS. Quantitative analysis of intestinal gas in patients with acute pancreatitis. Hepatobiliary Pancreat Dis Int 2012;11:314-8. [Crossref] [PubMed]
- Raghuwanshi S, Gupta R, Vyas MM, Sharma R. CT Evaluation of Acute Pancreatitis and its Prognostic Correlation with CT Severity Index. J Clin Diagn Res 2016;10:TC06-11. [PubMed]
- Kedia S, Sharma R, Bopanna S, Makharia G, Ahuja V. Predictive Model for Differentiating Crohn's Disease and Intestinal Tuberculosis: The story Is Incomplete Without Imaging. Am J Gastroenterol 2017;112:188-9. [Crossref] [PubMed]
- Savolainen R. Imaging of rectal cancer-key to treatment decisions. Duodecim 2016;132:1170-5. [PubMed]
- Viremouneix L, Monneuse O, Gautier G, Gruner L, Giorgi R, Allaouchiche B, Pilleul F. Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging 2007;26:331-8. [Crossref] [PubMed]
- Vriens PW, van de Linde P, Slotema ET, Warmerdam PE, Breslau PJ. Computed tomography severity index is an early prognostic tool for acute pancreatitis. J Am Coll Surg 2005;201:497-502. [Crossref] [PubMed]
- Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, Cohen R, Windsor A, Obichere A, Hansmann A, Novelli M, Halligan S, Taylor SA. Mural Inflammation in Crohn Disease: Location-Matched Histologic Validation of MR imaging Features. Radiology 2009;252:712-20. [Crossref] [PubMed]
- Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Multi-de-tector CT features of acute intestinal ischemia and their prognostic correlations. World J Radiol 2014;6:130-8. [Crossref] [PubMed]
- Kim JH, Ha HK, Sohn MJ, Shin BS, Lee YS, Chung SY, Kim PN, Lee MG, Auh YH. Usefulness of MR Imaging for diseases of the small intestine: comparison with CT. Korean J Radiol 2000;1:43-50. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Bradley EL 3rd. A clinically based classification system for acute pancretitis. Summary of the international symposium on Acute Panereatitis Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586-90. [Crossref] [PubMed]
- Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. J Surg Res 2006;135:18-26. [Crossref] [PubMed]
- Ahualli J. The Target Sign: bowel wall. Radiology 2005;234:549-50. [Crossref] [PubMed]
- Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics 2010;30:367-84. [Crossref] [PubMed]
- Ramalho M, Herédia V, Cardoso C, Matos AP, Palas J, De Freitas J, Semelka RC. Magnetic resonance imaging of small bowel Crohn's disease. Acta Med Port 2012;25:231-40. [PubMed]
- Finkelstone L, Wolf EL, Stein MW. Etiology of small bowel thickening on computed tomography. Can J Gastroenterol 2012;26:897-901. [Crossref] [PubMed]
- Ishikawa K, Idoguchi K, Tanaka H, Tohma Y, Ukai I, Watanabe H, Matsuoka T, Yokota J, Sugimoto T. Classification of acute pancreatitis based on retroperitoneal extension: application of the concept of interfascial planes. Eur J Radiol 2006;60:445-52. [Crossref] [PubMed]
- Lee SL, Ku YM, Rha SE. Comprehensive reviews of the interfascial plane of the retroperitoneum: normal anatomy and pathologic entities. Emerg Radiol 2010;17:3-11. [Crossref] [PubMed]
- Korobkin M, Silverman PM, Quint LE, Francis IR. CT of the extraperitoneal space: normal anatomy and fluid collections. AJR Am J Roentgenol 1992;159:933-42. [Crossref] [PubMed]
- Antonsen J, Tilma J. Images in clinical medicine. Mechanical small-bowel obstruction. N Engl J Med 2014;371:e12. [Crossref] [PubMed]
- Hussein M, Issa G, Muhsen S, Haydar A. Superior mesenteric arteriovenous fistula embolisation complicated by bowel ischaemia. BMJ Case Rep 2013;2013:bcr2013009521. [PubMed]
- Mao R, Liao WD, He Y, Ouyang CH, Zhu ZH, Yu C, Long SH, Chen YJ, Li ZP, Wu XP, Lv NH, Hu P, Chen M. Computed tomographic enterography adds value to colonoscopy in differentiating Crohn's disease from intestinal tuberculosis: a potential diagnostic algotithm. Endoscopy 2015;47:322-9. [Crossref] [PubMed]
- Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, Faingold R, Taylor G, Gerstle JT. Necrotizing enterocolitis: review of state- of- the-art imaging findings with pathologic correlation. Radiographics 2007;27:285-305. [Crossref] [PubMed]
- Gong J, Mei D, Yang M, Xu J, Zhou Y. Emergency CT of blunt abdominal trauma: experience from a large urban hospital in Southern China. Quant Imaging Med Surg 2017;7:461-8. [Crossref] [PubMed]
- Antonini F, Pezzilli R, Angelelli L, Macarri G. Pancreatic disorders in inflammatory bowel disease. World J Gastrointest Pathophysiol 2016;7:276-82. [Crossref] [PubMed]
- Schrum A, Scheer F, Andresen R. Colon-Cut-off-Sign in the CT-Scanogram – Evidence of Pancreatitis? J Clin Diagn Res 2015;9:TD01-2. [PubMed]
- Khan KH, Khan MF, Khan TJ. Gastric perforation without generalized peritonitis; A very rare complication after necrosectomy for necrotizing pancreatitis. Pak J Med Sci 2016;32:782-5. [Crossref] [PubMed]
- Nagpal AP, Soni H, Haribakhti S. Severe colonic complications requiring subtotal colectomy in acute necrotizing pancreatitis- A retrospective study of 8 patients. Indian J Surg 2015;77:3-6. [Crossref] [PubMed]
- Hua Z, Su Y, Huang X, Zhang K, Yin Z, Wang X, Liu P. Analysis of risk factors related to gastrointestinal fistula in patients with severe acute pancreatitis: a retrospective study of 344 cases in a single Chinese center. BMC Gastroenterol 2017;17:29. [Crossref] [PubMed]
- Wei AL, Guo Q, Wang MJ, Hu WM, Zhang ZD. Early complications after interventions in patients with acute pancreatitis. World J Gastroenterol 2016;22:2828-36. [Crossref] [PubMed]
- Bruno MJ. Dutch Pancreatitis Study Group. Improving the Outcome of Acute Pancreatitis. Dig Dis 2016;34:540-5. [Crossref] [PubMed]
- Boumitri C, Brown E, Kahaleh M. Necrotizing Pancreatitis: Current Management and Therapies. Clin Endosc 2017;50:357-65. [Crossref] [PubMed]
- Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, Coburn N, May GR, Pearsall E, McLeod RS. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016;59:128-40. [Crossref] [PubMed]
- Xiao B, Zhang XM, Tang W, Zeng NL, Zhai ZH. Magnetic resonance imaging for local complications of acute pancreatitis: A pictorial review. World J Gastroenterol 2010;16:2735-42. [Crossref] [PubMed]
- Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010;105:435-41. [Crossref] [PubMed]


