Identifying osteoporotic vertebral endplate and cortex fractures
Importance of identifying vertebral fracture (VF)
Osteoporosis is the most common metabolic bone disease, estimated to affect more than 200 million people worldwide (1). Osteoporosis is characterized by low bone mass and micro-architectural deterioration, which leads to bone fragility and consequent increase in fracture risk. It is often termed the “silent epidemic” because clinical factors (age, lifestyle, family history, diet) are not very sensitive markers of the condition, and quite often bone loss becomes apparent only after a typical fracture has occurred.
VFs are the most common osteoporotic fracture. A VF, after minor trauma, is a hallmark of osteoporosis. Thirty to fifty percent of women and 20–30% of men experience a VF at some points in their life (2). A single atraumatic VF can lead to the diagnosis of osteoporosis. According to the National Osteoporosis Foundation 2013 clinical guide, ‘‘A vertebral fracture is consistent with a diagnosis of osteoporosis, even in the absence of a bone density diagnosis, and is an indication for pharmacologic treatment with osteoporosis medication to reduce fracture risk’’ (1). In one study of osteoporotic fractures, only 39% of individuals with a VF had a World Health Organization diagnosis of osteoporosis by dual energy X-ray absorptiometry (DXA) at the spine, only 25% by DXA of the total hip (3). Prevalent VFs increase the risk of future vertebral and non-vertebral osteoporotic fracture independent of bone mineral density (BMD) (2).
It had been reported that spinal fractures are associated with increased mortality which at year-5 is nearly identical to that from femoral neck fractures (4), though it is not clear if this is cause-and-effect or if the fracture is simply a marker for frailty (3). A low-energy occult VF, when recognized, is a sufficient indication to initiate medical osteoporosis therapy independent of BMD results. Appropriate management of osteoporosis can reduce future fracture risk. It is important to identify and report VFs accurately and clearly, so that appropriate investigation and treatment can be instigated (5). Theoretically, by mitigating anterior compressive loads, the number of occult VFs that become clinically apparent might be reduced.
Osteoporotic VFs are frequently asymptomatic. The majority are morphometric (75%), that is, clinically silent without an incident of back pain, rather than with clinical symptoms (25%) (6). Incidental osteoporotic VFs are usually under-reported on radiograph and CT (7-10). The sensitivity of axial CT images in detecting these fractures is poor, sagittal reformation is recommended to improve the detection rate (10).
VFs have an important influence on prognosis and morbidity in the osteoporotic patient (11,12). The accurate and clear reporting of VFs is essential to ensure that patients with osteoporosis receive appropriate treatment. Radiologist has an important role in the diagnosis of this disease. Exclusion of other causes of VF is crucial, in particular malignant disease. Once the diagnosis of osteoporosis is made and initiation of therapy is planned, additional BMD measurements may be useful (5).
Radiographic definitions of osteoporotic vertebral facture
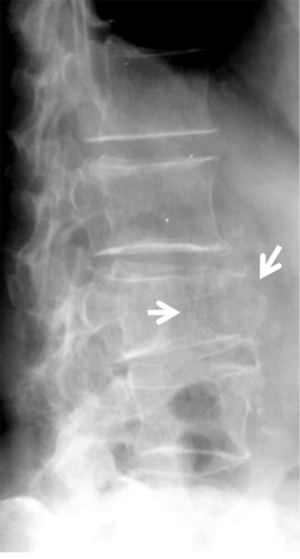
The radiographic signs of osteoporotic VF include: (I) loss of self-similarity between adjacent vertebrae; (II) loss of parallelism between adjacent end-plates; (III) end-plate disruption as they are impacted into the vertebral body; (IV) fractures of vertebral cortex or end-plate characterized by cortical discontinuities; (V) buckling of the vertebral cortex, especially anteriorly (13). A number of morphometrical and radiological methods for detecting osteoporotic VF have been proposed, but there is no consensus regarding the definition of VFs (5,13-16). We feel a combined approach based on standardized radiologic evaluation by experts aided by quantitative measurement to ensure the consistence is the most appropriate approach to detect and classify VF.
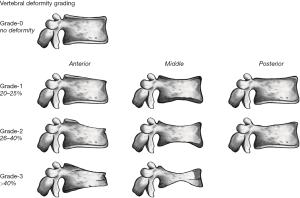
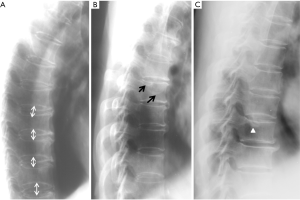
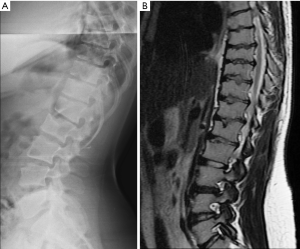
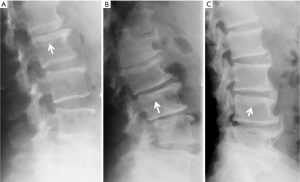
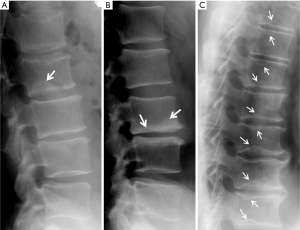
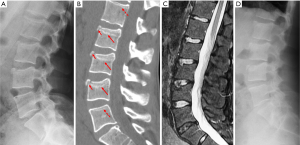
Since vertebrae generally fail by compression in axial loading, these fractures are often called vertebral compression fractures. The semi-quantitative (SQ) criteria proposed by Genant et al. is commonly used for identifying VF (17). According to its criteria, a normal vertebra is defined as grade 0; 20–25% reduction in anterior, middle, and/or posterior vertebral height and 10–20% in area is defined as grade 1 deformity; a 26–40% reduction in any height and 20–40% in area is defined as grade 2 deformity; and a >40% reduction in any height and area is regarded as grade 3 deformity vertebra (Figures 1-3). A grade 0.5 is used to designate a borderline deformed. Genant et al.’s SQ approach is applied to vertebrae T4 to L4 on the spine radiograph. Visualizing T1–T3 is often limited due to overlying of the shoulders and L5 due to overlying pelvis. Osteoporotic VF above T4 is also very rare. Vertebra L5 is also subject to considerable morphologic variation between individuals, such that very often vertebral anterior height measurement is much larger than posterior height (Figure 4). Genant et al. described the importance of loss of end-plate integrity as a characteristic of fractures but did not make diagnosis contingent on this observation.
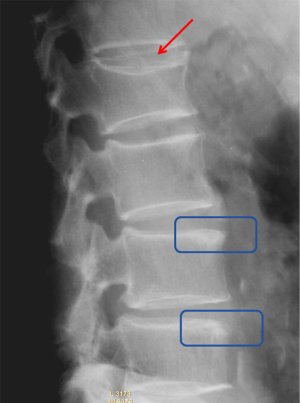
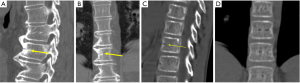
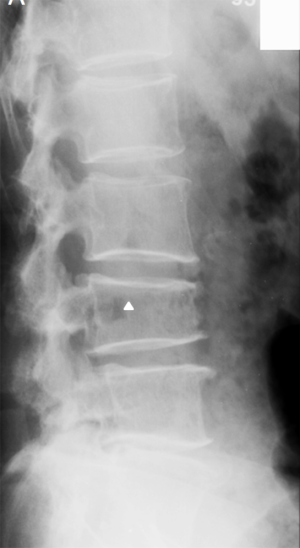
Historically, conventional radiography is used as the reference standard to identify a VF. More recently, DXA imaging with improved image quality is also used (5). Computed tomography (CT) and magnetic resonance imaging (MRI) are used for characterization (dating and differential diagnosis). In epidemiological studies, usually only lateral radiograph is taken. Nevertheless, a baseline set of both antero-posterior and lateral images help with diagnosis and follow-up. Lateral fractures may be difficult to identify from lateral radiograph. Serial radiographs of a patient should be viewed together in temporal order to accomplish a thorough and reliable analysis of all new fractures. Worsening of a VF, e.g., progression from grade 1 to 2, is usually considered to be equivalent to an incident fracture. Because a VF can be a permanent event that is present on follow-up radiographs, temporal blinding may not be useful. Most readers can identify a temporal sequence of films by new deformities as well as by progressive disc degeneration and osteophyte formation. High quality radiographs are important for reliable VF diagnosis. Diagnostic errors may be caused by technical shortcomings of image acquisition such as poor patient positioning (Figure 5).
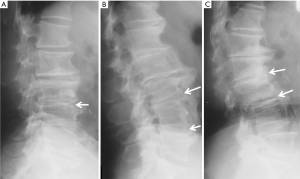
A linear correlation exists between both the number and severity of prevalent VFs and future VF risk (7). A “spinal deformity index (SDI)” can be calculated from SQ assessment as the sum of all grades assigned to the vertebrae divided by the number of the vertebrae evaluated (8). For each vertebra, a visual SQ of 0, 1, 2, or 3 is assigned for no fracture or mild, moderate, or severe fracture, respectively; and the SDI is calculated by summing the fracture grades of the 13 vertebrae from T4 to L4 (18). An increase in SDI could occur either due to a new VF or due to worsening of mild or moderate prevalent VFs.
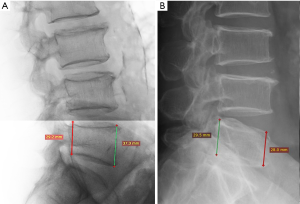
An experienced reader may estimate the severity of fracture according to the apparent degree of vertebral height reduction; but without the aid of direct measurements, the reduction in vertebral height cannot be determined with high accuracy, particularly when it is close to the cutoff values for grading of fracture. For example, the criterion of 20% to 25% reduction of vertebral height for identification of a grade 1 fracture may not be estimated with high accuracy and consistency. Therefore, while estimation of the degree of vertebral height reduction may be used for clinical diagnostic purpose; for epidemiological study and follow-up evaluation, we feel quantitative measurement is necessary to ensure the consistency and accuracy. It is expected that quantitative software tool will aid this aspect (19).
The Genant et al.’s SQ approach may bring equivocal diagnosis for grade 1 VF. Grade 1 VDs are often either false positive or deformities related to non-osteoporotic diseases of the spine, particularly in male subjects. Variation in vertebral height may occur in developmental abnormalities with or without degenerative changes. Some height loss is expected with ageing, due to compression of the intervertebral discs and postural changes. As age-related degenerative changes occur, the vertebral wedge angle increases. This case is particularly difficult with mild wedge fractures in the mid-thoracic region. Szulc et al. (20) suggested that at the level of thoracic kyphosis (T6–T9) that a cutoff of 30% for wedge deformities from T6 to T9 and of 25% for other deformities has a high specificity and a moderate sensitivity for identifying VDs related to low BMD in men.
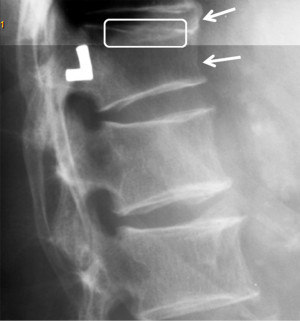
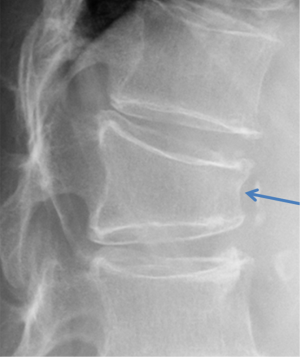
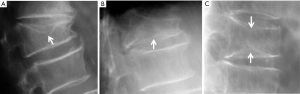
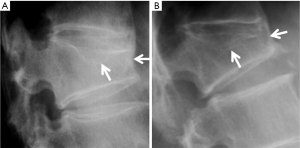
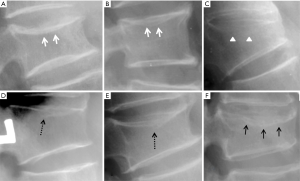
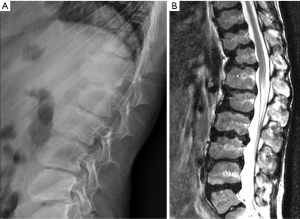
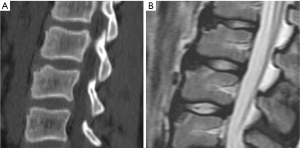
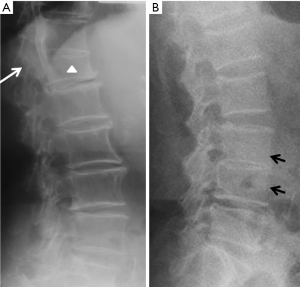
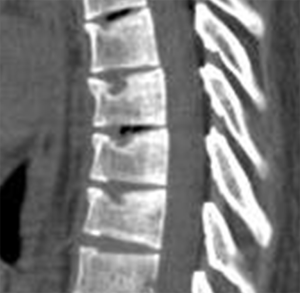
Vertebrae may fracture, generate fracture pain yet not result in measurable changes in radiographic configuration height or area (21). The point has been emphasized concerning the diagnosis of osteoporotic VFs as a fracture of the vertebral endplate or other cortex parts. Algorithm-Based Qualitative approach (ABQ) was developed with a focus on the identification of change in the vertebral endplate (Figures 6) (22,23). On a true lateral projection, the superior (or inferior) surface of the normal vertebra exhibits two lines; one line represents one side of the vertebral ring, and the second (more dense) line represents the central endplate superimposed on the opposite vertebral ring (22). The expected appearances of the central vertebral endplate in osteoporotic fracture are based on the assumption that because the center of the endplate within the vertebral ring is the weakest area, this will be the primary site of fracture. However, due to fan-like shape of X-ray beam, many vertebrae are not filmed with a perfect lateral projection.
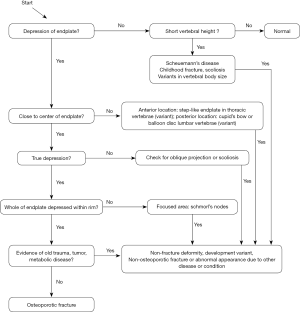
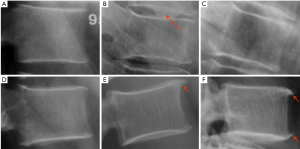
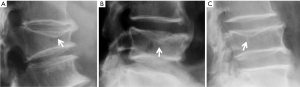
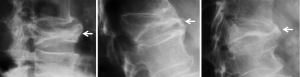
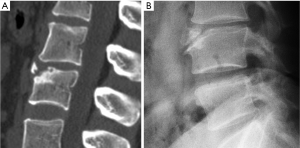
In addition to endplate, fracture can occur in anterior cortex without VD; or fractures deform the anterior vertebral cortex without endplate disruption. Some of them are classified by radiologist as buckling, dented, swollen and projecting types of fracture (Figures 7-9).
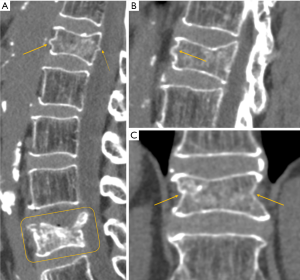
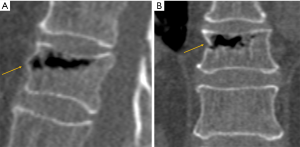
On conventional radiographs it may be difficult to determine the age of a fracture. Cortical disruption and impaction of trabeculae with increased density adjacent to the endplate are signs of more recent, acute fractures. Subacute fractures show additional callus formation along the endplate with increasing density. However, these signs are not always reliable, in particular in osteoporotic bone where the increase in density adjacent to the endplate may be minimal. In osteoporotic fractures, medullary signal changes on MRI depend on the age of the fracture. Acute fractures usually show edema with band-like pattern in the subchondral endplate. In chronic fractures, there is reversion to normal fat signal of bone marrow.
It has been reported that although radiologically apparent VFs are more likely to show as areas of increased isotope uptake on scintigraphy than uncrushed vertebrae, this did not depend on the age of the crush fracture (24). Isotope uptake by VF depend not so much upon the age of the VF but upon the extent of new bone formation. In osteoporosis cases, new bone formation can be at a low rate over an extended duration and in some cases of severe end stage osteoporosis, new bone formation may be almost absent. Therefore some acute/sub-acute VFs do not show as areas of increased isotope uptake on scintigraphy. On the other hand, some crushed vertebrae appear to take up diphosphonate for >2 years (24).
Although many fractures of moderate and severe grades are readily recognized, inevitably there will be findings that are not clear-cut, and in respect of which even “experts” may disagree. If clinical decision-making hinges on the diagnosis of a fracture, then comparison with previous images, a radionuclide scan, computed tomography (with sagittal reformation), and/or MRI may provide clearer evidence. Osteoporotic VFs are frequently located at the thoracolumbar junction where, owing to the overlying diaphragm, visualization may be difficult. Naturally, particularly attention should be paid to the thoracolumbar conjunction region and middle thoracic spine region (around T8 level).
This article aims to serve as a teaching material for physicians or researchers to identify vertebral endplate/cortex fracture (ECF). Emphasis is particularly dedicated to identifying vertebral ECF which may not be associated apparent vertebral body collapse. This article tries to avoid overlap or repeat many excellent reviews recently published on the topic of the osteoporotic spine fracture, such as references (5,13,25-29). It is in the accurate diagnosis of asymptomatic VFs that radiologists can make the most significant contribution to osteoporotic patient care.
Identifying osteoporotic VF
A vertebral body fracture after minor trauma is a hallmark of osteoporosis. Because the damage is limited to the anterior vertebral column in most cases, the fracture is usually stable and not associated with neurologic impairment.
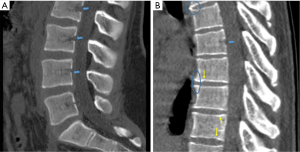
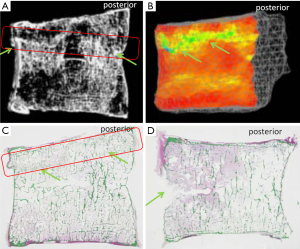
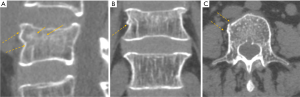
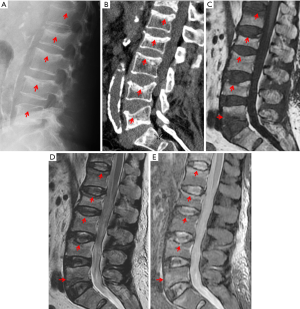
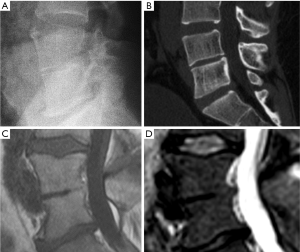
VF and their repair/healing occur frequently even in the absence of any appreciable radiographic change in vertebral shape (Figure 10). Microscopic fractures as shown by histology are common in radiographically normal vertebrae (31). Antonacci et al. (31) reported that of 24 adjacent vertebrae (in which fractures were not appreciated by specimen radiographs) of radiographic osteoporotic VF, 13 (54%) had histologic evidence of endplate fracture. Eight of the 24 (33%) showed evidence of anterior cortical fracture. True fractures of these bones occurred while not apparent radiographically. In the vertebra specimens with radiographic osteoporotic VF, 88% had endplate fractures histologically. And for those with endplate fracture by histologic criteria, 21% (5/24) had bone healing bridge the fracture site. In addition, in the vertebra adjacent to the radiographically fractured vertebra due to osteoporosis, the bony endplate was not continuous in 7 of the 24 (29%). Therefore we could consider microscopic trabecula fracture and repair, ECF without VD, and vertebral wedge/crush fracture a spectrum of presentations of compromised vertebral bone strength. Imaging modalities such as nuclear scintigraphy and MR are useful in identifying symptomatic VFs before diagnostic radiographic changes are seen.
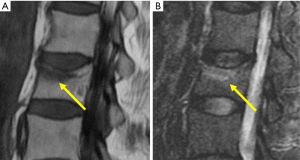
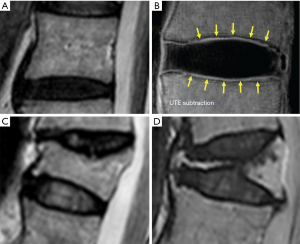
Fracturing vertebrae with pain, particularly recently fracturing vertebrae, may not be accompanied by radiographic findings (21). Transient vertebral marrow edema events that fail to meet a radiographic threshold for fracture and remain clinically silent are common. The altered MR vertebral marrow signal is not simply “bone edema”, but contain histopathologic trabecular fracture and repair in “unfractured” osteoporotic vertebrae. In these cases, radiology service should provide quick, cost-effective protocols for recognition of occult VF in urgent care areas such as a limited, sagittal STIR sequence MR imaging of the relevant spinal region of interest (Figure 11). However, MRI may not be a sensitive method for identifying ECF, unless specific techniques such as UTE (ultrashort TE) are employed (Figure 12). Endplate and cortical bone have very short T2* and appears as a signal void on images obtained with routine clinical pulse sequences. UTE technique allows acquisition of MR signals much earlier after excitation than with conventional sequences. As a result, MR signals from previously “invisible” tissues with very short T2/T2* values such as bone can now be acquired and imaged with high signal intensity. However, UTE method remains in research setting and not clinically available yet.
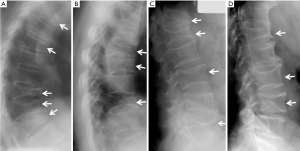
Radiographic appearances of ECFs are shown in Figures 13-25.
Radiographic artefacts and congenital variance, and non-fracture pathologies
Unexpected vertebral body contour with or without vertebral height loss may mimic the diagnosis of VF. When a congenital defect in vertebral formation occur, the vertebra, or part of it, does not develop properly, resulting in alterations such as agenesis, wedged vertebra, hemivertebra and butterfly vertebra. Some of these changes result in anterior wedging leading to a short vertebral height in adults. Carefully looking for fracture signs should be a critical step for VF diagnosis. Sometimes we may recognize these abnormalities as variants based on the radiograph, but sometimes CT, MRI or radiography follow-up is needed in order to reach an accurate diagnosis.
Radiographic artefacts due to X-ray beam projection
X-ray beam projection can cause many radiographic artefacts (Figure 26). These can usually be readily identified by experienced radiologists.
Development changes mimicking VF
Many developmental deformities or variants may mimic VF. Once a radiologist recognizes these developmental changes, usually a correct differential diagnosis can be made without many difficulties. Some common developmental deformities are listed below:
Anterior vertebral vascular groove
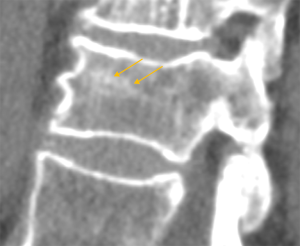
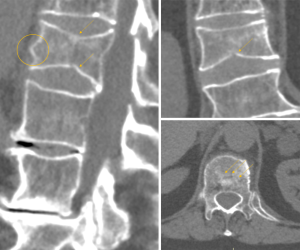
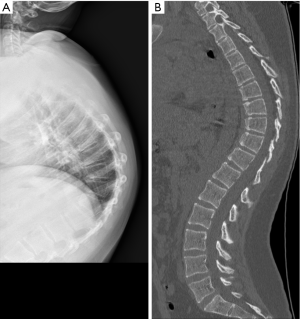
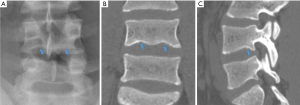
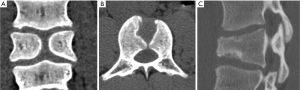
The anterior vertebral vascular groove is a thin, transverse linear indentation in anterior cortex, mainly seen in children, but also sometime visible in adults. Coronal CT reconstruction help to delineate the tubular structures corresponding to vessels from a linear vertebral cortex fracture (Figure 27).
Mucopolysaccharidosis
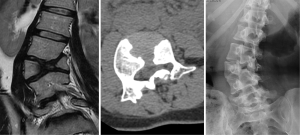
Mucopolysaccharidosis comprises a group of metabolic disorders related to lack or malfunctioning of lysosomal enzymes (33). The resulting abnormal storage of glycosaminoglycans affects bone and connective tissues, with thoracolumbar junction hypoplastic vertebrae and kyphosis (Figure 28) (33,34). Hurler Syndrome (mucopolysaccharidosis type I) usually presents with vertebrae showing anterior inferior beaking, and Morquio syndrome (mucopolysaccharidosis type IV) patients more frequently show anterior central vertebral body beaking and round vertebral bodies (33,34).
Spondyloepiphyseal dysplasia
Spondyloepiphyseal dysplasia is a well-defined, X-linked primary skeletal dysplasia that predominantly affects the spinal vertebral bodies and epiphyses during skeletal growth (35). These patients often develop early onset kyphoscoliosis and low back pain. Platyspondyly and vertebral hypoplasia are common radiologic findings. Imaging shows radiographic irregularity of vertebral endplates with bi-convex lower lumbar vertebral bodies (Figure 29). There is disc space narrowing and frequent calcification of the intervertebral discs.
Limbus vertebra
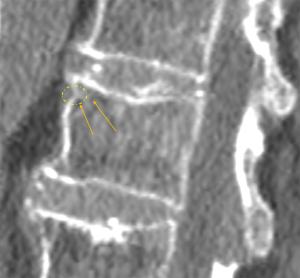
Limbus vertebrae results from an anterior (or posterior) intraosseous herniation of the nucleus pulposus and was first described in details by Neidner (36). In early adolescence, before the ring apophyses ossify, there are small radiolucent defects at the anterior corners of the vertebral bodies that interrupt the vertebral endplates. The clue to differentiate it from a fracture is the preservation of the height and convexity of the rest of the vertebral endplates (37). The apophyseal rings ossification occurs around the age of 10–15. After that, the apophyseal rings fuse with vertebral body. Before the maturation of spine, if nucleus pulposus herniates between the unfused ring apophyses and the growing end plate, the fusion between both structures is prevented and the ring fragment is isolated from the rest of the vertebral body (Figures 30,31). This is known as limbus fracture and must not be confounded with fracture or inflammatory process (38)
Wedge shaped vertebra
Physiologic vertebral development, being development or degenerative, may also lead to vertebral wedging and mimic VF (Figure 32). In normal children and adults, the vertebral body is anteriorly wedged from T1 through L2 (peak at T7), non-wedged at L3, and posteriorly wedged at L4 to L5 (peak at L5). For diagnosing VF, a careful search for fractured/deformed endplate and cortical line is important, though we admit to clearly identity endplate/cortex fracture may not be possible in all cases due to the inherent spatial resolution limitation of radiograph.
Idiopathic kyphosis and scoliosis usually associate with wedging of the vertebral bodies secondary to abnormal and non-uniform mechanical load of the vertebral bodies. In both cases no structural abnormalities are found that justify the abnormal curvatures. Although in case of kyphosis some authors consider any vertebral wedging to indicate Scheuermann’s disease (39,40), when no another vertebral abnormalities are associated to wedging, such as end-plate irregularity or Schmorl nodes, it is more appropriate to consider this as idiopathic kyphosis (Figures 33,34) (41,42).
Short height vertebra
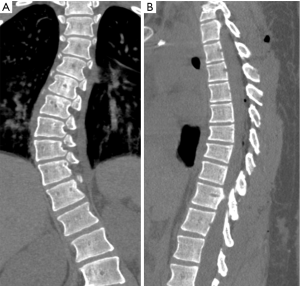
Some adults have vertebrae that have short anterior height in developmentally small thoracic vertebrae. Those deformities with reduction of height but which lack visible depression of the endplate or other ECF signs represent the non-osteoporotic deformity of short height vertebra (Figure 35).
‘Stair-Step’ shaped vertebral endplate
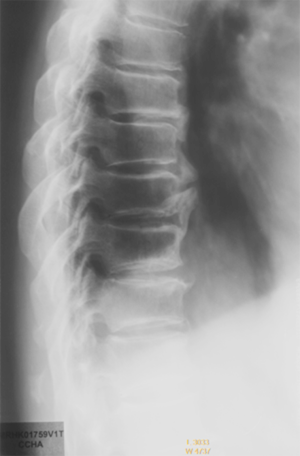
‘Stair-step’ shaped vertebral endplate is a common sign seen on thoracic spine radiograph (Figure 36).
Block vertebrae
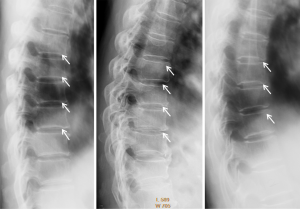
Block vertebrae occur when there is improper segmentation of the vertebrae (Figure 37). The adjacent vertebrae fuse through their intervertebral discs and also through other intervertebral joints. It can lead to blocking or stretching of the exiting nerve roots from that segment, and can increase stress on the inferior and the superior intervertebral joints. The sacrum is a normal block vertebra. The vertebral ring apophyses of the fused vertebral endplates do not develop properly leading to thinning of the vertebral contour at the junction of both vertebrae. The antero-posterior diameter at the level of the fusion is small, leading to a concave anterior contour (the wasp’s waist sign) (Figure 38). It has been also described in congenital abnormalities of the spine, such as Klippel Feil syndrome (43).
Cupid’s bow
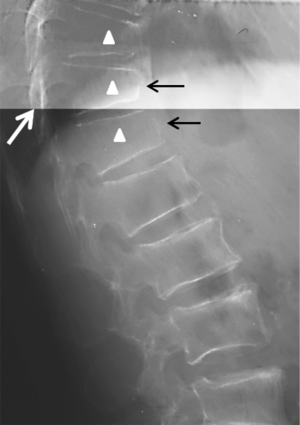
Cupid’s bow is a para-sagittal concave curvature in the inferior endplates of the vertebral bodies (some author argue that it can also be at superior and inferior endplates). Although the degree of concavity varies, the configuration of the Cupid’s bow is relatively constant and is characterized by a concavity located in the posterior third of the vertebral body with a relatively smooth curvature, which has a shallow angle anteriorly and a steep curvature posteriorly. It has been suggested that the thin layer of cartilage in the vertebral endplate is absent in the para-sagittal region, while instead a layer of calcium was found within this area (Figures 39,40). Owing to the absence of the cartilaginous plate in the parasagittal portions of the diskovertebral junction, the bone growth of the vertebral body is hindered at this particular region, resulting in the appearance of Cupid’s bow concavities (44-46). Cupid’s bow is a developmental phenomenon without pathologic implications. Cupid’s bow is usually seen at the inferior endplate at L3, L4 and L5, but it can also occur at all levels of the lumbar spine and the lower thoracic spine

Scheuermann’s disease
Scheuermann’s disease was initially described in 1920, by Holger Scheuermann, as a rigid thoracic and thoracolumbar kyphosis associated with vertebral body wedging in late childhood (47). Scheuermann’s disease is a common cause of hyperkyphosis of the thoracic and thoracolumbar spine during adolescence. After idiopathic scoliosis, it is the most common disorder in patients with a deformed spine. This condition is characterized by vertebral body wedging, vertebral endplate irregularity, diminished anterior vertebral growth, Schmorl’s nodes, narrowing of the intervertebral disc spaces and premature disks degeneration (Figures 41,42). Sørensen described radiographic criteria for typical Scheuermann disease include anterior wedging greater than 5° in at least three adjacent vertebral bodies (48).
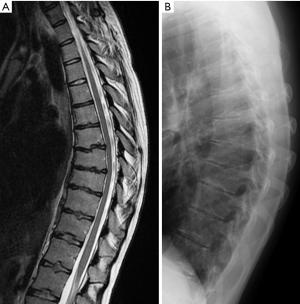
The disease starts during adolescence when the growth plates are still open and it most likely represents an osteochondrosis. The anterior vertebral body wedging is secondary to the increased anterior forces due to the first occurring kyphosis. Another radiological sign correlated with Scheuermann disease pathogenesis is the length of the sternum. A smaller length of the sternum has been associated with Scheuermann disease (49). When apophyseal changes affect the thoracolumbar or lumbar spine, especially when there is associated pain, the diagnosis of lumbar Sheuermann’s disease can be made. It is not typically associated with significant clinical kyphosis (50). The natural history of Scheuermann kyphosis remains controversial, with conflicting reports as to the severity of pain and physical disability (51).
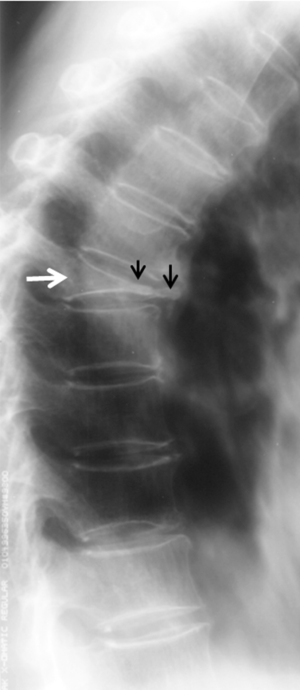
Calvé’s disease
Calvé’s disease is a rare cause of “vertebra plana” in which the flattening of a single vertebral body shows the typical ‘‘coin on edge’’ appearance (Figure 43). In 1925 Calvé described a condition he described as ‘‘osteochondritis’’ of a vertebral body. He also laid down the criteria of the disease: (I) total collapse of only one vertebra; (II) no involvement of the intervertebral disc; (III) the intervertebral space wider than normal at least by one-third; and (IV) increased density of the involved vertebra (52). In 1927, Buchman suggested the name “vertebra plana” for this condition (53). In 1954, Compere suggested Calvé’s disease was caused by eosinophilic granuloma (54). Many later other publications and a large number of cases presented support Compere’s views (Figure 44) (55).
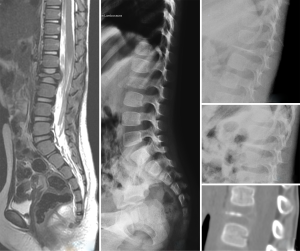
Notochordal remnant
The notochord induces the formation of the neural plate and serves as a model on which the axial skeleton will develop. Progressive chondrification of the vertebral body displaces the notochord into the intervertebral space, where it forms the nucleus pulposus of the intervertebral disc (Figure 45). Persistence of residual notochordal tissue in the vertebral body is a rare event that may mimic a VF fracture in radiograph (56,57). Severe deformity and a lack of cortical line fracture help the differential diagnosis.
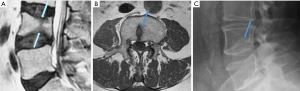
Butterfly vertebra
Butterfly vertebra is an uncommon congenital anomaly of the spine. Between the third and sixth weeks of gestation the mesenchymal somites become in two cartilaginous nuclei, rather than posteriorly fuse into a single vertebral body. Failure of the fusion of the two halves of the vertebral body leads to persistent remnants of the notochord in-between resulting in the butterfly VD (Figures 46,47). Symmetric may be an asymptomatic, but may be a cause of painful congenital scoliosis when asymmetric (58). Severe deformity and a lack of cortical surface fracture help the differential diagnosis.
Degenerative changes
Radiologists are generally familiar with the degenerative changes seen on spine imaging (Figure 48). Schmorl’s node may have the potential to cause confusion with endplate fracture. A Schmorl’s node is characterized by displacement of a portion of the intervertebral disc into the vertebral body due to disruption of the cartilaginous endplate (Figure 49) (59). The Schmorl’s node forms a depression, leading to discontinuity and an irregular contour of the vertebral endplate, and can involve any portion of the vertebral endplate (Figures 50,51).

Posttraumatic non-osteoporotic deformity after compression fractures
A reduction in bone density, accentuated secondary or vertical trabeculations giving a striated appearance and sharply outlined cortical end-plates are radiologic signs of osteoporosis and may be helpful for the differentiation of osteoporotic fractures from non-osteoporotic fractures. If the available clinical history is inadequate then the radiographic appearance may be useful. In posttraumatic deformities, usually the diameter of the vertebrae is enlarged and there may be are substantial, secondary, degenerative changes with osteophyte formation (Figure 52). Callus formation tends to be more substantial in traumatic fracture.
However, in some cases post-traumatic fractures are difficult to differentiate form osteoporotic collapse in absence of positive history and associated hematoma. Osteoporotic VFs also often result from minor trauma.
VFs due to malignant diseases
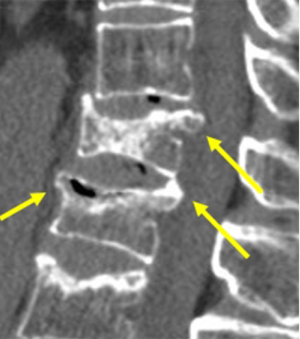
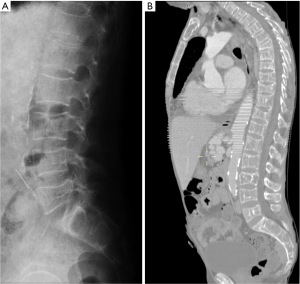
The most important differential diagnosis for osteoporotic VF is malignant diseases, particularly metastatic bone diseases or multiple myeloma. The differential diagnosis between osteoporotic and malignant pathologic fractures can be sometimes difficult in elderly subjects. Fractures that are located above the T7 level, present with a soft tissue mass, osseous destruction and fractures of the posterior part of the vertebral body in conventional radiograph, most likely have a malignant etiology (Figure 53). Retropulsion of the posterosuperior or inferoposterior margin of the vertebral body toward the canal is suggestive of benignancy and typical of burst fracture that affects the posterior vertebral wall of the vertebral body (Figure 54). A concave posterior border of the vertebra is more likely a sign of a benign osteoporotic fracture, in particular if there is some retropulsion of bony parts into the spinal canal, while a convex posterior border suggests malignant disease (5). Morphologic changes suggest malignancy when a convex posterior cortex of the vertebral body is seen due to a mass effect or epidural and/or paravertebral masses. Nevertheless, abnormal involvement of the pedicle in osteoporotic compression fractures has been reported (62). Therefore, when pedicle involvement is noted in VFs and when it is the only sign, the diagnosis of malignant pathologic fracture cannot be assumed.
A sign of benign disease is the fluid sign, which is demonstrated in acute vertebral compression fractures that show bone marrow edema. It is found adjacent to the fractured endplates and exhibits signal intensity isointense to that of cerebrospinal fluid (63). This finding is reversible as fracture healing occurs. Its size remains unchanged for 2–4 months and then can gradually returns to normal. This is an additional sign of osteoporosis and rarely occurs in metastatic fractures (64). Other findings highly suggestive of acute osteoporotic vertebral collapse are ‘‘intravertebral vacuum cleft sign’’, features of vertebral osteonecrosis (Figure 55). Vertebral osteonecrosis is an infrequent condition, consequence of ischemic phenomena in the vertebral end plate and subchondral bone following compression fractures.
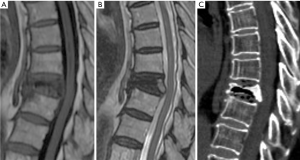
As a search methodology and for appreciation of the spinal tumor involvement, MRI is the diagnostic tool of choice. MRI can play an important role in this task due to its capacity to detect fractures before radiograph morphologic changes appear (65). Fat-suppression or short tau inversion recovery (STIR) sequences are often necessary and increase the detection of metastatic lesions, mainly in cases of highly fatty bone marrow. Collapsed vertebral body with normal bone marrow signal intensity indicates a lack of marrow replacement and suggests acute or subacute osteoporotic fractures. Convex vertebral margins may be present secondary to intra-vertebral tumor expansion. Asymmetric invasion of paravertebral or epidural mass are characteristic of malignancy (Figure 56). When the differential diagnosis is not possible, imaging guided biopsy may be indicated.
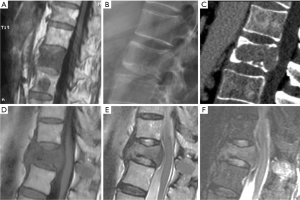
Excessive bone resorption is a characteristic feature of multiple myeloma and is responsible for bone weakening. On conventional radiograph, a diffuse osteoporosis and focal osteolytic lesions are most frequently observed, separately or in association. In typical cases of multiple myeloma, there are multiple lytic lesions along with osteopenia and VF is associated with a soft tissue component (Figure 57). VF happens in 50–70% of patients with multiple myeloma. It may lead to spinal cord compression in 15% of the patients (66,67). Many vertebral compression fractures in patients with multiple myeloma appear benign on MR imaging (Figure 58). In addition, their site distribution is similar to that of compression VFs in patients with osteoporosis. On imaging multiple myeloma’s appearance may be misleading, simulating an osteoporotic compression VF in about 67% of the cases and the fractures may present different state of evolution with 38% of the patients having only benign appearing VFs (68).
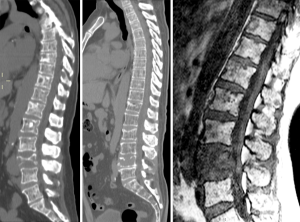
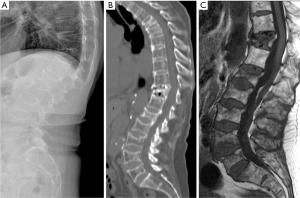
VFs due to other causes of skeleton general fragility
Low energy fractures can be due to other secondary causes causing general fragility of the skeleton, such as osteomalacia, osteogenesis imperfecta, hyperparathyroidism, chronic kidney disease, sickle cell anemia, thalassemia (Figure 59), Paget’s disease, etc. Recognising the features of these diseases is important for a correct diagnosis. The detailed differentiation diagnosis discussion is beyond the scope of this review.
Summary
This pictorial essay illustrates the importance of endplate and cortical surface analysis for detecting VF to increase diagnostic accuracy. Future studies are necessary to evaluate how to incorporate different definitions of osteoporotic VF into practice. As opposed to the VFs with VD, the clinical predictive values of ECF without VD remain to be further validated. We hope this pictorial will aid future studies on epidemiology of mild ECF and their clinical implications.
When reporting the epidemiology of osteoporotic VF, the prevalence and new incidence rate are constrained by the methodology and criteria used. Imaging methods, such as Xtreme CT, which has resolution for bone close to histology level, if applied for spine, would certainly report high VF prevalence in subjects with osteoporotic. Same would be the case for MRI to be used to identify new osteoporotic VF incidence. The methodology applied in practice should be clinically relevant and provide evidence for clinical management.
Acknowledgements
The authors thank Professor Richard Eastell at The University of Sheffield for his encouragement during the course of preparing this paper, and his critical comments. The content of this manuscript does not necessarily reflect the viewpoints of Professor Eastell.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359-81. [Crossref] [PubMed]
- Ross PD. Clinical consequences of vertebral fractures. Am J Med 1997;103:30S-42S. [Crossref] [PubMed]
- Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. Osteoporotic Fractures Research Group. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 2003;18:1947-54. [Crossref] [PubMed]
- Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ 3rd. Population based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001-5. [Crossref] [PubMed]
- Link TM, Guglielmi G, van Kuijk C, Adams JE. Radiologic assessment of osteoporotic vertebral fractures: diagnostic and prognostic implications. Eur Radiol 2005;15:1521-32. [Crossref] [PubMed]
- Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE. Fracture Intervention Trial Research Group. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 2005;20:1216-22. [Crossref] [PubMed]
- Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of Vertebral Fracture in Clinical Setting. Osteoporos Int 2000;11:577-82. [Crossref] [PubMed]
- Kim N, Rowe BH, Raymond G, Jen H, Colman I, Jackson SA, Siminoski KG, Chahal AM, Folk D, Majumdar SR. Underreporting of vertebral fractures on routine chest radiography. AJR Am J Roentgenol 2004;182:297-300. [Crossref] [PubMed]
- Majumdar SR, Kim N, Colman I, Chahal AM, Raymond G, Jen H, Siminoski KG, Hanley DA, Rowe BH. Incidental vertebral fractures discovered with chest radiography in the emergency department. Arch Intern Med 2005;165:905-9. [Crossref] [PubMed]
- Williams AL, Al-Busaidi A, Sparrow PJ, Adams JE, Whitehouse RW. Under-reporting of osteoporotic vertebral fractures on computed tomography. Eur J Radiol 2009;69:179-83. [Crossref] [PubMed]
- Broy SB. The Vertebral Fracture Cascade: Etiology and Clinical Implications. J Clin Densitom 2016;19:29-34. [Crossref] [PubMed]
- Harrison RA, Siminoski K, Vethanayagam D, Majumdar SR. Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone Miner Res 2007;22:447-57. [Crossref] [PubMed]
- Lentle B, Trollip J, Lian K. The radiology of osteoporotic vertebral fractures redux. J Clin Densitom 2016;19:40-7. [Crossref] [PubMed]
- Oei L, Koromani F, Rivadeneira F, Zillikens MC, Oei EH. Quantitative imaging methods in osteoporosis. Quant Imaging Med Surg 2016;6:680-98. [Crossref] [PubMed]
- Griffith JF. Identifying osteoporotic vertebral fracture. Quant Imaging Med Surg 2015;5:592-602. [PubMed]
- Oei L, Rivadeneira F, Ly F, Breda SJ, Zillikens MC, Hofman A, Uitterlinden AG, Krestin GP, Oei EH. Review of radiological scoring methods of osteoporotic vertebral fractures for clinical and research settings. Eur Radiol 2013;23:476-86. [Crossref] [PubMed]
- Genant HK, Wu CY, Van Kuijk C, Nevitt M. Vertebral fracture assessment using a SQ technique. J Bone Miner Res 1993;8:1137-48. [Crossref] [PubMed]
- Sauer P, Leidig G, Minne HW, Duckeck G, Schwarz W, Siromachkostov L, Ziegler R. Spine deformity index (SDI) versus other objective procedures of vertebral fracture identification in patients with osteoporosis: a comparative study. J Bone Miner Res 1991;6:227-38. [Crossref] [PubMed]
- Oei L, Ly F, El Saddy S, Makurthou AA, Hofman A, van Rooij FJ, Uitterlinden AG, Zillikens MC, Rivadeneira F, Oei EH. Multi-functionality of computer-aided quantitative vertebral fracture morphometry analyses. Quant Imaging Med Surg 2013;3:249-55. [PubMed]
- Szulc P, Munoz F, Marchand F, Delmas PD. Semiquantitative evaluation of prevalent vertebral deformities in men and their relationship with osteoporosis: the MINOS study. Osteoporos Int 2001;12:302-10. [Crossref] [PubMed]
- McKiernan FE. The broadening spectrum of osteoporotic vertebral fracture. Skeletal Radiol 2009;38:303-8. [Crossref] [PubMed]
- Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 2004;15:887-96. [Crossref] [PubMed]
- Ferrar L, Jiang G, Clowes JA, Peel NF, Eastell R. Comparison of densitometric and radiographic vertebral fracture assessment using the algorithm-based qualitative (ABQ) method in postmenopausal women at low and high risk of fracture. J Bone Miner Res 2008;23:103-11. [Crossref] [PubMed]
- Hordon LD, Francis RM, Marshall DH, Smith AH, Peacock M. Are scintigrams of the spine useful in vertebral osteoporosis? Clin Radiol 1986;37:487-9. [Crossref] [PubMed]
- Guermazi A, Mohr A, Grigorian M, Taouli B, Genant HK. Identification of vertebral fractures in osteoporosis. Semin Musculoskelet Radiol 2002;6:241-52. [Crossref] [PubMed]
- Bazzocchi A, Guglielmi G. Vertebral Fracture Identification. Semin Musculoskelet Radiol 2016;20:317-29. [Crossref] [PubMed]
- Guglielmi G, Diacinti D, van Kuijk C, Aparisi F, Krestan C, Adams JE, Link TM. Vertebral morphometry: current methods and recent advances. Eur Radiol 2008;18:1484-96. [Crossref] [PubMed]
- McKiernan FE. Violet Fox: A Clinical View of Vertebral Fractures. J Clin Densitom 2016;19:35-9. [Crossref] [PubMed]
- Adams JE. Opportunistic Identification of Vertebral Fractures. J Clin Densitom 2016;19:54-62. [Crossref] [PubMed]
- Imai K. Analysis of vertebral bone strength, fracture pattern, and fracture location: a validation study using a computed tomography-based nonlinear finite element analysis. Aging Dis 2015;6:180-7. [Crossref] [PubMed]
- Antonacci MD, Mody DR, Rutz K, Weilbaecher D, Heggeness MH. A histologic study of fractured human vertebral bodies. J Spinal Disord Tech 2002;15:118-26. [Crossref] [PubMed]
- Hwang D, Kim S, Abeydeera NA, Statum S, Masuda K, Chung CB, Siriwanarangsun P, Bae WC. Quantitative magnetic resonance imaging of the lumbar intervertebral discs. Quant Imaging Med Surg 2016;6:744-55. [Crossref] [PubMed]
- Scaramuzzo L, Perisano C, Leone A, Graci C, Spinelli MS, Di Giacomo G, Venanzi E, Schiavone Panni A, Maccauro G. Skeletal modifications in mucopolysaccharidoses: an overview. J Biol Regul Homeost Agents 2012;26:139-44. [PubMed]
- Grainger RG, Allison DJ. Grainger and Allison's diagnostic radiology, a textbook of medical imaging. Churchill Livingstone (2009). ISBN:0443064326.
- Iceton JA, Horne G. Spondylo-epiphyseal dysplasia tarda: the X-linked variety in three brothers. J Bone Joint Surg Br 1986;68:616-9. [PubMed]
- Neidner F. Zur Kenntnis der normalen und pathologischen Wirbekoerperrandleisten. Fortschr Roentgenstr 1932;46:628.
- Jaremko JL, Siminoski K, Firth GB, Matzinger MA, Shenouda N, Konji VN, Roth J, Sbrocchi AM, Reed MH, O'Brien MK, Nadel H, McKillop S, Kloiber R, Dubois J, Coblentz C, Charron M, Ward LM. Canadian STOPP Consortium National Pediatric Bone Health Working Group. Common normal variants of pediatric vertebral development that mimic fractures: a pictorial review from a national longitudinal bone health study. Pediatr Radiol 2015;45:593-605. [Crossref] [PubMed]
- Ghelman B, Freiberger RH. The limbus vertebra: an anterior disc herniation demonstrated by discography. AJR Am J Roentgenol 1976;127:854-5. [Crossref] [PubMed]
- Bradford DS, Moe JH, Montalvo FJ, Winter RB. Scheuermann’s kyphosis and roundback deformity: Results of Milwaukee brace treatment. J Bone Joint Surg (Am) 1974;56:740-58. [Crossref] [PubMed]
- Taylor TC, Wenger DR, Stephen J, Gillespie R, Bobechko W. Surgical management of thoracic kyphosis in adolescents. J Bone Joint Surg (Am) 1979;61:496-503. [Crossref] [PubMed]
- Stagnara, P. Les deformations du rachis. Paris: Masson, 1985.
- Platero D, Luna JD, Pedraza V. Juvenile kyphosis: effects of different variables on conservative treatment outcome. Acta Orthop Belg 1997;63:194-201. [PubMed]
- Nguyen VD, Tyrrel R. Klippel-Feil syndrome: patterns of bony fusion and wasp-waist sign. Skeletal Radiol 1993;22:519-23. [Crossref] [PubMed]
- Dietz GW, Christensen EE. Normal “Cupid’s bow” contour of the lower lumbar vertebrae. Radiology 1976;121:577-9. [Crossref] [PubMed]
- Ramirez H, Navarro JE, Bennett WF. “Cupid’s bow” contour of the lumbar vertebral endplates detected by computed tomography. J Comput Assist Tomogr 1984;8:121-4. [Crossref] [PubMed]
- Chan KK, Sartoris DJ, Haghighi P, Sledge P, Barrett-Connor E, Trudell DT, Resnick D. Cupid's bow contour of the vertebral body: evaluation of pathogenesis with bone densitometry and imaging-histopathologic correlation. Radiology 1997;202:253-6. [Crossref] [PubMed]
- Scheuermann H. Kyphosis dorsalis juvenilis. Z Orthop Chir 1921;41:4.
- Sørensen KH. Scheuermann’s juvenile kyphosis: clinical appearances, radiography, etiology and prognosis. Copenhagen: Munksgaard, 1964.
- Fotiadis E, Grigoriadou A, Kapetanos G, Kenanidis E, Pigadas A, Akritopoulos P, Samoladas E. The role of sternum in the etiopathogenesis of Scheuermann disease of the thoracic spine. Spine 2008;33:E21-4. [Crossref] [PubMed]
- Blumenthal SL, Roach J, Herring JA. Lumbar Scheuermann’s. A clinical series and classification. Spine 1987;12:929-32. [Crossref] [PubMed]
- Bezalel T, Carmeli E, Been E, Kalichman L. Scheuermann's disease: current diagnosis and treatment approach. J Back Musculoskelet Rehabil 2014;27:383-90. [Crossref] [PubMed]
- Calvé J. A localized affection of the spine suggesting osteochondritis of the vertebral body, with the clinical aspects of Pott’s disease. J Bone Joint Surg (Am) 1925;7:41-9.
- Buchman J. Osteochondritis of the vertebral body. J Bone Joint Surg (Am) 1927;9:55-66.
- Compere EL, Johnson WE, Coventry MB. Vertebra plana (Calvé ’s disease) due to eosinophilic granuloma. J Bone Joint Surg (Am) 1954;36-A:969-80. [Crossref] [PubMed]
- Shisha T, Kiss S, Varga PP, Bucsi L, Pap K, Szoke G. Osteochondritis (Calvé’s disease) of a vertebral body--a rare form of vertebra plana. Eur Spine J 2006;15:377-83. [Crossref] [PubMed]
- Musgrove J. Persistence of the notochord in the human subject. J Anat Physiol 1891;25:386-9. [PubMed]
- Oner AY, Akpek S, Tokgoz N. Persistent Notochordal Canal Mimicking Compression Fracture: A Case Report. Acta Radiol 2006;47:875-7. [Crossref] [PubMed]
- Hopkins RM, Abbott JH. Congenital ‘butterfly vertebra’ associated with low back pain: a case report. J Man Manip Ther 2015;23:93-100. [Crossref] [PubMed]
- Resnick D, Niwayama C. Intravertebral disk herniations: cartilaginous (Schmorl’s) nodes. Radiology 1978;126:57-65. [Crossref] [PubMed]
- Siriwanarangsun P, Statum S, Biswas R, Bae WC, Chung CB. Ultrashort time to echo magnetic resonance techniques for the musculoskeletal system. Quant Imaging Med Surg 2016;6:731-43. [Crossref] [PubMed]
- Ruiz Santiago F, Tomás Muñoz P, Moya Sánchez E, Revelles Paniza M, Martínez Martínez A, Pérez Abela AL. Classifying thoracolumbar fractures: role of quantitative imaging. Quant Imaging Med Surg 2016;6:772-84. [Crossref] [PubMed]
- Ishiyama M, Fuwa S, Numaguchi Y, Kobayashi N, Saida Y. Pedicle involvement on MR imaging is common in osteoporotic compression fractures. AJNR Am J Neuroradiol 2010;31:668-73. [Crossref] [PubMed]
- Baur A, Stabler A, Arbogast S, Duerr HR, Bartl R, Reiser M. Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. Radiology 2002;225:730-5. [Crossref] [PubMed]
- Cicala D, Briganti F, Casale L, Rossi C, Cagini L, Cesarano E, Brunese L, Giganti M. Atraumatic vertebral compression fractures: differential diagnosis between benign osteoporotic and malignant fractures by MRI. Musculoskelet Surg 2013;97 Suppl 2:S169-79. [Crossref] [PubMed]
- Jung HS, Jee WH, McCauley TR, Ha KY, Choi KH. Discrimination of metastatic from acute osteoporotic compression spinal fractures with MR imaging. Radiographics 2003;23:179-87. [Crossref] [PubMed]
- Lecouvet FE, Vande Berg BC, Michaux L, Jamart J, Maldague BE, Malghem J. Development of vertebral fractures in patients with multiple myeloma: does MRI enable recognition of vertebrae that will collapse? J Comput assit Tomog 1998;22:430-6.
- Spiess JL, Adelstein DJ, Hines JD. Multiple myeloma presenting with spinal cord compression. Oncology 1988;45:88-92. [Crossref] [PubMed]
- Lecouvet FE, Vande Berg BC, Maldague BE, Michaux L, Laterre E, Michaux JL, Ferrant A, Malghem J. Vertebral compression fractures in Multiple Myeloma. Part I. Distribution and Appearance at MR Imaging. Radiology 1997;204:195-9. [Crossref] [PubMed]


