Acute myocardial infarction and stroke secondary to valve thrombosis following transcatheter aortic valve replacement—what can happen when antiplatelet agents are stopped
Introduction
An 84-year-old woman was hospitalised for a fall with subsequent agitation and vomiting. She had a past medical history of coronary artery bypass grafting performed 18 years ago. In the 8 months prior to her admission, she underwent transcatheter aortic valve replacement (TAVR) for severe aortic stenosis (AS). Post procedure, the patient had been instructed to take dual antiplatelet therapy (DAPT) for 6 months but had stopped these herself earlier than the recommendation.
On physical examination she was noted to have dysphasia, right upper limb weakness and hypotonia. An electrocardiography (ECG) showed dynamic inferior ST elevation and her serum troponin level was elevated.
A cardiac gated computed tomography (CT) of the aorta and coronary arteries was performed to exclude an aortic dissection and assess the coronary arteries. The CT demonstrated thrombus extending from the TAVR into the right coronary artery (Figures 1,2). A CT brain and magnetic resonance imaging (MRI) showed an acute infarct in the left parietal lobe. (Figures 3,4)
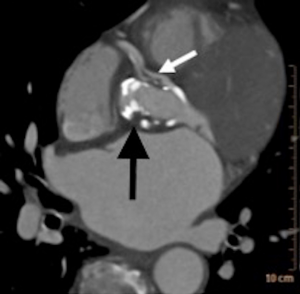
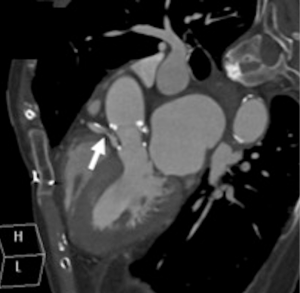
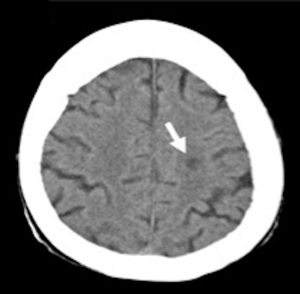
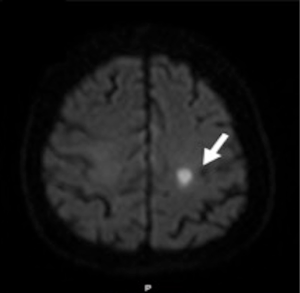
She was treated with antiplatelets and anticoagulation with intravenous (IV) heparin. Her ECG changes and neurological deficits resolved subsequently.
Discussion
AS is the commonest valvular heart disease leading to surgery or catheter intervention in Europe (1). Surgical aortic valve replacement is recommended for symptomatic severe AS (2). Older persons often have multiple comorbidities, rendering them at high surgical risk. TAVRs, bioprosthetic valves inserted into the aortic valve position percutaneously are a treatment option for these patients (2).
The rate of thromboembolism among bioprosthetic aortic valve patients is 1.9% per patient-year despite anti-thrombotic therapy (2), underscoring the importance of the prevention of thromboembolism in TAVR patients. Current guidelines on post TAVR anti-thrombotic therapy, however, are not uniform and the strength of recommendations is not strong. The 2014 AHA/ACC guideline on valvular heart disease states that lifelong aspirin plus clopidogrel for 6 months “may be reasonable” (Class IIb, level of evidence C) (3). The 2017 AHA expert consensus document on TAVR recommends standard anti-thrombotic therapy should be clopidogrel 75 mg daily for 3–6 months with lifelong aspirin 75–100 mg daily (4). ESC guidelines recommend a combination of low-dose aspirin and a thienopyridine in the first 3–6 months after TAVR, followed by a lifelong single antiplatelet; although single anti-platelet therapy may be considered in those at high risk of bleeding (1). Position statements from the Canadian Cardiovascular Society (CCS) (DAPT for 1–3 months) (5) and ACCF/AATS/SCAI/STS (DAPT for 3–6 months) (6) give similar recommendations.
Whilst guideline bodies all recommend lifelong aspirin after TAVR, the recommended duration of DAPT varies, making it unclear as to what the optimal duration of DAPT is. The role of new oral anticoagulants post-TAVR is currently being explored. It is possible that these may be superior to antiplatelet therapy in the prevention of valve thrombosis.
There are currently no specific guidelines on perioperative antiplatelet therapy in TAVR patients, making the management of patients undergoing procedures difficult. Bridging heparin is only recommended in patients with mechanical prosthetic valves (1,3,7). We are unaware of any centre that recommends the use of bridging heparin for TAVR patients who need suspension of antiplatelet therapy. A recent study has, however, shown that anticoagulation with novel oral anticoagulants and warfarin, but not DAPT was effective in the prevention and treatment of subclinical leaflet thrombosis, which itself was associated with increased rates of transient ischemic attacks (TIAs) and strokes (8). Currently, we recommend a balance of risks and reintroduction of the antiplatelet therapy as soon as possible
Bioprosthetic valve thrombosis, as in our case, is uncommon. The first-line treatment of bioprosthetic valve thrombosis is anticoagulation with a vitamin K antagonist and/or unfractionated heparin (1).
In summary, this case highlights the potential effects of stopping antiplatelets agents in TAVR and emphasises the importance of post TAVR antithrombotics. A balance must be made between risks of bleeding and thrombosis. Until antiplatelets can be restarted, there is no role for preoperative bridging heparin either via subcutaneous or intravenous routes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [PubMed]
- Heras M, Chesebro JH, Fuster V, Penny WJ, Grill DE, Bailey KR, Danielson GK, Orszulak TA, Pluth JR, Puga FJ, Schaff HV, Larsonkeller JJ. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol 1995;25:1111-9. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [Crossref] [PubMed]
- Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S, Lee JC, Ruiz CE, Vassileva CM. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2017;69:1313-46. [Crossref] [PubMed]
- Webb J, Rodés-Cabau J, Fremes S, Pibarot P, Ruel M, Ibrahim R, Welsh R, Feindel C, Lichtenstein S. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol 2012;28:520-8. [Crossref] [PubMed]
- Holmes DR Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200-54. [Crossref] [PubMed]
- Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Lüscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C. Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383-431. [Crossref] [PubMed]
- Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, Rami T, Israr S, Fontana G, de Knegt M, Fuchs A, Lyden P, Trento A, Bhatt DL, Leon MB, Makkar RR. RESOLVE; SAVORY Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389:2383-92. [Crossref] [PubMed]


