How liver pathologies contribute to T1rho contrast require more careful studies
Liver T1rho has been proposed as an imaging biomarker for liver fibrosis (1-5). In the recent study of Xie et al. (6,7), 18 healthy subjects, 18 patients with fatty liver, and 18 patients with liver fibrosis had T1rho MR imaging performed. The diagnosis of liver fibrosis was based on serologic evidence of hepatitis B or C virus infection and liver stiffness criteria by ultrasonic elastography. Mean liver T1rho values in fibrotic group (52.6±6.8 ms) were significantly higher than those of healthy subjects (44.9±2.8 ms, P<0.001) and fatty liver group (45.0±3.5 ms, P<0.001), while mean liver T1rho values were similar between healthy subjects and fatty liver group (P=0.999). However, four liver fibrosis cases (4/18, 22.2%) had liver T1rho overlapped with those of control subjects (the first figure of Xie et al.’s paper) (7). Since there was no histologic diagnosis of liver fibrosis for the patients, it is possible that these four cases actually did not have fibrosis; however, it is more likely that these patients did have liver fibrosis, but their liver T1rho was not measured higher than healthy subjects’ liver.
It is well known that liver diseases can be associated with increased liver iron deposition (8). Ludwig et al. (9) studied tissue iron in 447 cirrhotic livers, and increased hepatic iron concentration was found in 20.3% of their cases. They concluded that iron overload is common in many types of nonbiliary cirrhosis. The hemosiderosis of affected liver occur rapidly once cirrhosis has developed, and cirrhosis alone may cause iron accumulation. It is probable that in Xie et al.’s study there was increased liver iron in these four cases, and the associated liver T2* shortening complicated T1rho measurement. We have seen such phenomenon in our own unpublished clinical cases.
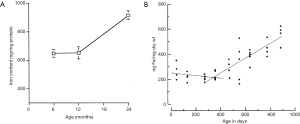
Among healthy subjects, our recent paper shows women have higher physiological liver T1rho than men; and younger subjects have higher physiological liver T1rho than older subjects with this trend being stronger in women than in men (10). Iron concentration in healthy subjects’ liver is also known to be higher in men than in women, and higher in older subjects compared with younger subjects (Figure 1) (11-13). Animal studies also demonstrated aging is associated with increased liver iron concentration (Figure 2) (14,15).

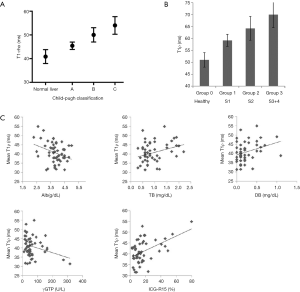
Previous studies show elevated T1rho is positively correlated with the severity of liver fibrosis/cirrhosis (Figure 3) (1-5). Elevated T1rho was correlated with the amount of collagen deposition in biliary duct ligation induced liver fibrosis (1). However, fiber tissue is not necessarily associated with higher T1rho inherently (16), as shown in intervertebral disc degeneration studies (17,18). Liver fibrosis, depending on its causes, is associated with a number of complicated pathological processes, including steatosis, hepatocellular ballooning, and inflammation (19,20). Inflammation involves presence of inflammatory cells, including lymphocytes, eosinophils and neutrophils, near ballooned hepatocytes. How these processes contribute to the elevated T1rho measurement deserve careful investigation, which can be answered partially by careful animal model studies. In an imperfect rat study reported by us (21), it can be seen that acute edema of liver caused by CCl4 intoxication was not a dominant contributor of T1rho increase. After the withdrawal of CCl4 stimulus, it seems that the lowering of elevated liver T1rho was faster than the resolve of fiber tissue deposition in rat liver.
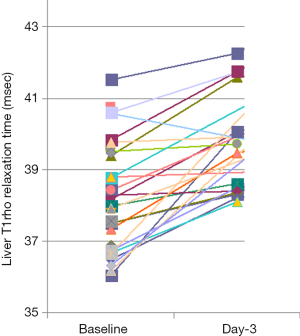
One note is the wide inter-subject variation of physiological T1rho, not only seen in healthy subjects (11), but also in male rats of similar age (Figure 4) (5). One potential explanation for Figure 4 is that these rats have diverse liver iron concentrations, but co-contributors of other sources are also possible and should be further investigated.
We can hypothesize that T1rho can differentiate liver steatosis (no T1rho elevation with fat suppressed sequence) from steatohepatitis (with T1rho elevation due to inflammation and fibrosis). The work of Xie et al. (6,7) suggests T1rho can differentiate simple fatty liver from liver pathologies involving inflammation and fibrosis.
With the recent progresses made in diffusion imaging (Figure 5) (22), it is possible that multi-parametric MRI approach, such as a combination of T1rho corrected with iron/T2*contribution and intravoxel incoherent motion (IVIM) imaging will allow early diagnosis of nonalcoholic steatohepatitis and liver fibrosis.
Acknowledgements
Funding: This study was partially supported by a grant from the Research Grants Council of Hong Kong SAR (Project No. 476313).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wang YX, Yuan J, Chu ES, Go MY, Huang H, Ahuja AT, Sung JJ, Yu J. T1rho MR imaging is sensitive to evaluate liver fibrosis: an experimental study in a rat biliary duct ligation model. Radiology 2011;259:712-9. [Crossref] [PubMed]
- Allkemper T, Sagmeister F, Cicinnati V, Beckebaum S, Kooijman H, Kanthak C, Stehling C, Heindel W. Evaluation of fibrotic liver disease with whole-liver T1rho MR imaging: a feasibility study at 1.5 T. Radiology 2014;271:408-15. [Crossref] [PubMed]
- Singh A, Reddy D, Haris M, Cai K, Rajender Reddy K, Hariharan H, Reddy R. T1ρ MRI of healthy and fibrotic human livers at 1.5 T. J Transl Med 2015;13:292. [Crossref] [PubMed]
- Takayama Y, Nishie A, Asayama Y, Ushijima Y, Okamoto D, Fujita N, Morita K, Shirabe K, Kotoh K, Kubo Y, Okuaki T, Honda H. T1 ρ Relaxation of the liver: A potential biomarker of liver function. J Magn Reson Imaging 2015;42:188-95. [Crossref] [PubMed]
- Koon CM, Zhang X, Chen W, Chu ES, San Lau CB, Wang YX. Black blood T1rho MR imaging may diagnose early stage liver fibrosis: a proof-of-principle study with rat biliary duct ligation model. Quant Imaging Med Surg 2016;6:353-63. [Crossref] [PubMed]
- Xie S, Li Q, Zhuo Z, Zhang Y, Cheng Y, Shen W. Liver T1rho detects liver fibrosis without impact of fatty liver in patients with chronic hepatitis B: a prospective study. Available online: http://dev.ismrm.org/2017/2029.html
- Xie S, Li Q, Zhuo Z, Zhang Y, Cheng Y, Shen W. Impact of Liver Fibrosis and Fatty Liver on T1rho Measurements: A Prospective Study. Korean J Radiol 2017;18:898-905. [Crossref]
- Nelson JE, Klintworth H, Kowdley KV. Iron metabolism in Nonalcoholic Fatty Liver Disease. Curr Gastroenterol Rep 2012;14:8-16. [Crossref] [PubMed]
- Ludwig J, Hashimoto E, Porayko MK, Moyer TP, Baldus WP. Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology 1997;112:882-8. [Crossref] [PubMed]
- Wáng YXJ, Deng M, Lo GG, Liang D, Yuan J, Chen W. Breath-hold black-blood T1rho mapping improves liver T1rho quantification in healthy volunteers. Acta Radiol 2017. Epub ahead of print. [Crossref] [PubMed]
- Weinfeld A, Lundin P, Lundvall O. Significance for the diagnosis of iron overload of histochemical and chemical iron in the liver of control subjects. J Clin Pathol 1968;21:35-40. [Crossref] [PubMed]
- Schwenzer NF, Machann J, Haap MM, Martirosian P, Schraml C, Liebig G, Stefan N, Häring HU, Claussen CD, Fritsche A, Schick F. T2* relaxometry in liver, pancreas, and spleen in a healthy cohort of one hundred twenty-nine subjects-correlation with age, gender, and serum ferritin. Invest Radiol 2008;43:854-60. [Crossref] [PubMed]
- Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J 2000;140:98-104. [Crossref] [PubMed]
- Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev 1998;102:1-13. [Crossref] [PubMed]
- Massie HR, Aiello VR, Banziger V. Iron accumulation and lipid peroxidation in aging C57BL/6J mice. Exp Gerontol 1983;18:277-85. [Crossref] [PubMed]
- Wáng YX, Zhang Q, Li X, Chen W, Ahuja A, Yuan J. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg 2015;5:858-85. [PubMed]
- Wang YX, Zhao F, Griffith JF, Mok GS, Leung JC, Ahuja AT, Yuan J. T1rho and T2 relaxation times for lumbar disc degeneration: an in vivo comparative study at 3.0-Tesla MRI. Eur Radiol 2013;23:228-34. [Crossref] [PubMed]
- Gübitz R, Lange T, Gosheger G, Heindel W, Allkemper T, Stehling C, Gerss J, Kanthak C, Schulte TL. Influence of Age, BMI, Gender and Lumbar Level on T1ρ Magnetic Resonance Imaging of Lumbar Discs in Healthy Asymptomatic Adults. Rofo 2017. Epub ahead of print. [Crossref] [PubMed]
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010;5:145-71. [Crossref] [PubMed]
- Brunt EM. Histopathology of non-alcoholic fatty liver disease. Clin. Liver Dis 2009;13:533-44. [Crossref] [PubMed]
- Zhao F, Wang YX, Yuan J, Deng M, Wong HL, Chu ES, Go MY, Teng GJ, Ahuja AT, Yu J. MR. T1ρ as an imaging biomarker for monitoring liver injury progression and regression: an experimental study in rats with carbon tetrachloride intoxication. Eur Radiol 2012;22:1709-16. [Crossref] [PubMed]
- Wáng YX, Deng M, Li YT, Huang H, Leung JCS, Chen W, Lu PX. A Combined Use of Intravoxel Incoherent Motion MRI Parameters Can Differentiate Early-Stage Hepatitis-b Fibrotic Livers from Healthy Livers. SLAS Technol 2017. Epub ahead of print. [Crossref] [PubMed]