Endovascular management of arterial injuries after blunt or iatrogenic renal trauma
Introduction
The kidney is the most common injured genitourinary organ in case of external abdominal trauma (1). The major mechanisms that generate renal injuries are blunt, iatrogenic during an interventional procedure, intraoperative (2,3). Most injuries due to blunt trauma consist of lower-grade, non-life-threatening injuries. Renal injuries may be divided into renal laceration, renal contusion, and renal vascular injury (2-4). Iatrogenic renovascular injuries have increased due to the increasing number of interventional procedures such as renal artery angioplasty or stenting, percutaneous procedures such as biopsy, nephrostomy, nephro-ureterolithotomy, and nephron-sparing surgery (5-8). Possible radiological findings in iatrogenic vascular lesions are arteriovenous fistula, pseudoaneurysm, arterial dissection, or contrast extravasation. Most of these lesions heal spontaneously. However, they may result in massive bleeding, life-threatening hematuria or in deterioration of renal function, requiring immediate treatment (6-8). Nowadays, conservative or minimally invasive approaches are increasingly favoured over surgery, including transcatheter arterial embolization (TAE) and the placement of stents/stent grafts (2-16). Endovascular management is a well-established nonsurgical approach for renal arterial injuries, with many advantages, such as rapid recovery, short hospital stay and early resumption of physical activities. The aim of this article is to describe the role and technical aspects of endovascular interventions in the management of arterial injuries after blunt or iatrogenic renal trauma.
General technical considerations
Precise mapping of the vascular bed, including the aorta and its branches, and a selective study of the involved kidney must be performed to plan the therapeutic strategy. A catheter sheath introducer is used systematically. In patients with atheroma or tortuous arteries, using a long sheath (35 to 45 cm) with the tip positioned just downstream from the ostium of the renal arteries facilitates iterative catheterization. The use of 4 or 5 Fr conventional catheters and hydrophilic guidewires facilitates superselective catheterization and these devices make it possible to use microcatheters that are inserted co-axially into the approach catheter (5-7).
Considering that the choice of embolization material is important for efficient treatment of vascular injuries, the material should be chosen according to the site, size and the flow pattern of the vessels to be occluded, material availability, and the experience of the interventional radiologist (10). Percutaneous angiography with selective coil embolization is the initial treatment of choice in renal arterial bleeding. Superselective embolization as distally as possible is mandatory, at least at the level of the interlobar arteries, to keep parenchymal loss to a minimum. This can usually be achieved using microcatheters and microcoils as embolic agents. The main disadvantage of coil embolization is that usually more than one coil is required for adequate occlusion, which increases the cost and time of the procedure. For those reasons, liquid embolic agents such as n-butyl cyanoacrylate (NBCA) glue or Onyx® may be very useful. Cyanoacrylates offer the advantages of low viscosity for easy injection through small or tortuous catheters. The use of NBCA glue is particularly of interest in hemodynamically unstable patients and in cases of underlying coagulopathy, because it provides faster and better hemostasis than other embolic agents (7-10).
Blunt renovascular lesions
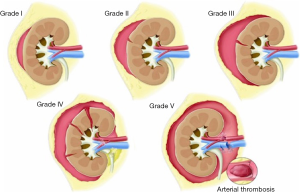
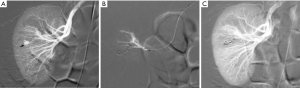
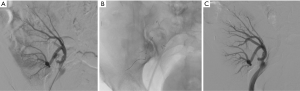
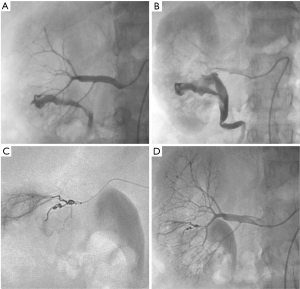
Renal injuries occur in approximately 1–5% of all patients with trauma (11). They are commonly identified on computed tomography (CT) imaging and graded according to the American Association for the Surgery of Trauma (AAST) classification system into five different grades (Figure 1): grade 1 = renal contusion or nonexpanding subcapsular hematoma without a parenchymal laceration, grade 2 = nonexpanding perirenal hematoma or a renal cortex laceration (<1 cm) without urinary extravasation, grade 3 = renal cortex laceration (>1 cm) and no urinary extravasation, grade 4 = renal cortical laceration extending into the collecting system (as noted by contrast extravasation) or a segmental renal artery or vein injury (noted by a segmental parenchymal infarct) or main renal artery or vein injury with a contained hematoma, and grade 5 = shattered kidney, avulsion of the renal pedicle, or thrombosis of the main renal artery (2). Operative vs. non-operative management of traumatic renal injuries has been debated for many years. Traditionally, high-grade renal injuries were treated with surgical exploration or nephrectomy. However, given advances in CT imaging and the minimally invasive techniques, non-operative management has become the standard of care (16). Indications for TAE following trauma vary from institution to institution. In cases of penetrating trauma, TAE is often a first-line alternative to surgery. For blunt trauma, the relative role of expectant management, embolization, and surgery may depend upon the grade of injury and overall patient status (4). Typically, embolization is performed to manage lower-grade renal injuries and penetrating injuries that are identified on CT imaging (Figure 2). The use of endovascular therapy in the context of grade 5 renal trauma and hemodynamic instability remains controversial, with conflicting reports of complete success or complete failure (16-19). However, TAE is increasingly performed at many institutions for high-grade renal injuries. A variety of clinical criteria have been proposed as key factors that predict need for renal embolization, including AAST grade, mechanism of injury, patient clinical stability, and concomitant visceral injuries, as it have multiple CT findings, such as active arterial bleeding, perirenal hematoma rim size, and disruption of Gerota’s fascia. Surgery should be performed in only three indications: life-threatening bleeding, renal pedicle avulsion (grade V), and an expanding non-contained retroperitoneal hematoma (2,16). TAE may prevent nephrectomy in up to 78% and 83% of grade IV and V renal injuries, respectively (4). However, for those same grade IV and V injuries many need a second endovascular intervention to treat recurrent bleeding. Based on these recommendations, diagnostic angiography and embolization have become a first-line therapeutic approach in the management of traumatic renal injuries. The two primary endovascular therapies which are offered by interventional radiology are embolization and stenting of injured renal arteries. Superselective arterial embolization can be employed in cases of renal arterial laceration, arteriovenous fistula, and pseudoaneurysm with different embolic agents (20-25). With respect to the management of renal arterial dissections, recent studies have supported the use of endovascular stent placement to treat traumatic intimal tears resulting in dissection and luminal stenosis (Figures 3,4) (9,15). In case of proximal arterial rupture, covered stents may be used.

Iatrogenic renovascular lesions
Injury to the lower back, and particularly stab wounds, percutaneous biopsies of the kidney and nephron-sparing surgery can cause vascular lesions that may give rise to various complications (5,8,11). Embolization is the best therapeutic option in such cases.
In the acute phase
In the hours that follow the trauma, it may not be possible to stop the bleeding, which may result in a state of shock. Emergency arteriography will very often reveal the injured vessels and active extravasation. It is quite simple to occlude the artery that feeds the damaged region using inert particles, resorbable material, glue or microcoils according to the morphology of the lesions (Figure 5). Hemostasis can generally be achieved. The few reported incidences of recurrent bleeding occurred when resorbable material was used (10). In these cases, the bleeding was stopped by repeat embolization. The renal infarction that can result from such interventions is often invisible or minor, as can be noted on surveillance examinations. This technique is only slightly aggressive and it preserves the renal parenchyma (7).
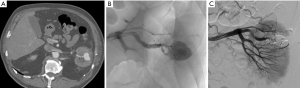
Large parenchymal false aneurysm into the left kidney after nephron-sparing surgery in a 58-year-old patient. (A) CT scan showing the false aneurysm into the left kidney; (B) superselective catheterization of the feeding artery and false aneurysm; (C) results after selective embolization of the aneurysmal sac and feeding artery with Onyx® demonstrating complete exclusion of the lesion with small cortical infarct.
In the post-acute period
The main problem in the post-acute period is persistent hematuria through the creation of an arteriourinary fistula (Figure 6). This very frequently occurs following renal biopsy, and it generally closes spontaneously within a few days. Should it persist for more than a week, it may complicate recovery. Percutaneous treatment consists of occluding the abnormal vessel responsible for the hematuria (5). The blood losses are generally small and these generally concern the peripheral arteries. Embolization can be easily done using inert particles or more often one or two microcoils. Bedsores may appear towards the tenth day. The clot that blocks the puncture site may sometimes detach causing sudden severe hemorrhage, which may be particularly serious if the patient is no longer under medical supervision. As is the case in the acute phase, emergency embolization is particularly useful as it stops the bleeding and prevents complications (7,8).
Massive hematuria with hypovolemic shock 2 hours after performing percutaneous kidney biopsy in a 56-year-old woman. (A,B) Presence of high-flow arteriocaliceal fistula on emergency renal angiography with rapid opacification of urinary cavities; (C) microcoil embolization of two abnormal vessels that were responsible for hematuria; (D) complete occlusion of fistula and cessation of bleeding are seen on post-embolization angiogram.
Delayed complications
Arteriovenous fistulas and false aneurysms are quite common, and their incidence increases with the number of percutaneous interventions, renal biopsies and surgical procedures. Arteriovenous fistulas occur following almost 15% of renal biopsies, but only 4% persist after several months. The vast majority heal spontaneously (8,11). Arteriovenous fistulas may also occur following nephrectomy if the entire vascular pedicle is ligated en bloc. The decision whether or not to repair this type of lesion depends on the blood flow through the fistula (heart failure, arterial hypertension), the risk of rupture in the case of large false aneurysms, and recurrent hematuria. In certain cases, fistulas are repaired to allow new renal biopsies to be performed. The morphology of the fistula must be carefully studied before embolization (Figure 7). The communication between the artery and the vein, or the false aneurysm when present should be the main target of the occlusion procedure. The principal technique is the deployment of metallic coils (Figure 8). An occlusion plug can sometimes be delivered into the feeding artery (10). Non-communicating false aneurysms are usually occluded using coils. In most cases, it is possible to preserve the downstream vascular bed and almost all of the renal parenchyma. Embolization is currently the treatment of choice for such fistulas. There is no doubt that the results of embolization are far superior to those obtained with surgery. The incidence of complications is low for experienced teams (7,11).
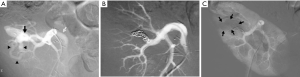
Progressive arterial hypertension in a 43-year-old patient one year after percutaneous renal allograft biopsy. (A) Selective arterial angiogram: there is large arteriovenous fistula in upper-pole segmental branch of transplanted renal artery and pseudoaneurysm (black arrow) with marked arteriovenous shunting (arrowheads) and early venous filling (white arrow). Note absence of nephrogram; (B) control angiogram after selective embolization of afferent artery with 0.035’’ coils: complete occlusion of pedicular aneurysm and fistula, and improvement in nephrogram; (C) post-embolization angiogram (parenchymal phase): renal infarction is seen in less than 10% of renal parenchyma (arrows).
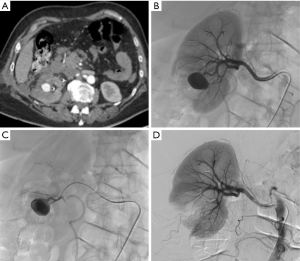
Bleeding from a pseudoaneurysm of the right kidney 3 weeks after nephron-sparing surgery. (A) CT scan showing the false aneurysm into the parenchyma; (B,C) selective and superselective angiograms of the right renal artery confirming the large vascular lesion; (D) angiography after superselective microcoil embolization of the feeding artery. Note only small parenchymal ischemia.
Complications
Pain
Depending on the volume of the involved parenchyma, the pain usually starts during the embolization and it may last 1 to 5 days. There is very little pain when treating arteriovenous fistulas. Patients must receive appropriate treatment with analgesics before, during and after the procedure (4).
Arterial hypertension
Transient increases in the arterial blood pressure are frequent and they may last for up to 24 hours. There is a risk of permanent arterial hypertension when there is a remnant of ischemic, but not infarcted renal tissue (7).
Kidney failure
Performing examination with agents that contain iodine may cause deterioration of the renal function. The patient must be appropriately hydrated before any embolization (13).
Post-embolization syndrome
This syndrome is almost inevitable in cases of destroying tissue and this syndrome includes nausea, vomiting, fever and abdominal pain. As a rule, the severity depends on the volume of the infarcted tissue. Paralytic ileus is often associated with the syndrome. Biological examinations will show hyperleukocytosis. The syndrome generally resolves with symptomatic treatment in a few days (7,18).
Hematuria
Moderate hematuria may occur following embolization. This is related to hemorrhagic infarction and it generally resolves within 24 to 48 hours.
Infections
There is a risk that latent infections may flare up, which means interventionalists need to be familiar with the patient’s medical record. Cytobacteriological examination of the patient’s urine must be systematically performed before the intervention, except for emergent procedures: a current urinary infection that is not controlled by appropriate treatment is a contra-indication for TAE. Small air bubbles have been found on the post-intervention CT images of patients with infection. They occur between the third and sixteenth day after the renal embolization. These air bubbles do not necessarily indicate an abscess, but they may correspond to normal aseptic infarction (7,8).
Non-target embolization
This is always possible whatever the material used. There is a notable risk of pulmonary embolization due to coils, permanent inert particles or glue in case of arteriovenous fistula. A microcoil can be recovered using a lasso snare or by surgery (10).
Other complications
As with any endovascular procedure, embolization and stenting carry a risk of hematoma at the entry point, arterial dissection, thrombosis, arterial rupture, distal embolism, anaphylactic reactions, shock, volume overload and kidney failure.
Conclusions
In conclusion, within the past few decades, TAE has become first-line therapy for renal trauma in lower AAST grade injuries and is increasing in prevalence for higher-grade renal injuries. TAE also represents safe and effective acute interventions for arterial bleeding control in the context of iatrogenic renovascular injuries, and could potentially reduce relevant morbidity and mortality rates. Hemodynamic stabilization of the patient should be considered the main purpose of TAE. For any indication, RAE should be discussed in a multidisciplinary team approach to establish clinical goals and expectations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Titton RL, Gervais DA, Boland GW, Mueller PR. Renal trauma: radiologic evaluation and percutaneous treatment of nonvascular injuries. AJR Am J Roentgenol 2002;178:1507-11. [Crossref] [PubMed]
- Santucci RA, McAninch JW, Safir M, Mario LA, Service S, Segal MR. Validation of the American Association for the Surgery of Trauma organ injury severity scale for the kidney. J Trauma 2001;50:195-200. [Crossref] [PubMed]
- Mani NB, Kim L. The role of interventional radiology in urologic tract trauma. Semin Intervent Radiol 2011;28:415-23. [Crossref] [PubMed]
- Ramaswamy RS, Darcy MD. Arterial Embolization for the Treatment of Renal Masses and Traumatic Renal Injuries. Tech Vasc Interv Radiol 2016;19:203-10. [Crossref] [PubMed]
- Loffroy R, Abualsaud B, Delgal A, Guiu B, Kermarrec I, Michel F, Cormier L, Mousson C, Majbri N, Rebibou JM, Cercueil JP, Krausé D. Role of percutaneous arterial embolization in renal pathology. Prog Urol 2010;20:161-71. [Crossref] [PubMed]
- Bittenbinder EN, Reed AB. Advances in renal intervention for trauma. Semin Vasc Surg 2013;26:165-9. [Crossref] [PubMed]
- Loffroy R, Rao P, Kwak BK, Ota S, De Lin M, Liapi E, Geschwind JF. Transcatheter arterial embolization in patients with kidney diseases: an overview of the technical aspects and clinical indications. Korean J Radiol 2010;11:257-68. [Crossref] [PubMed]
- Loffroy R, Guiu B, Lambert A, Mousson C, Tanter Y, Martin L, Cercueil JP, Krausé D. Management of post-biopsy renal allograft arteriovenous fistulas with selective arterial embolization: immediate and long-term outcomes. Clin Radiol 2008;63:657-65. [Crossref] [PubMed]
- Lee JT, White RA. Endovascular management of blunt traumatic renal artery dissection. J Endovasc Ther 2002;9:354-8. [Crossref] [PubMed]
- Loffroy R, Guiu B, Cercueil JP, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol 2009;7:250-63. [Crossref] [PubMed]
- Ierardi AM, Floridi C, Fontana F, Duka E, Pinto A, Petrillo M, Kehagias E, Tsetis D, Brunese L, Carrafiello G. Transcatheter embolisation of iatrogenic renal vascular injuries. Radiol Med 2014;119:261-8. [Crossref] [PubMed]
- Loffroy R.. N-Butyl Cyanoacrylate Glue: The Best Hemostatic Embolic Agent for Patients with Acute Arterial Bleeding. Cardiovasc Intervent Radiol 2017;40:1290-1. [Crossref] [PubMed]
- Ierardi AM, Duka E, Lucchina N, Floridi C, De Martino A, Donat D, Fontana F, Carrafiello G. The role of interventional radiology in abdominopelvic trauma. Br J Radiol 2016;89:20150866. [Crossref] [PubMed]
- Lorelli DR, Kralovich KA, Seguin C. The impact of pre-existing end-stage renal disease on survival in acutely injured trauma patients. Am Surg 2001;67:693-6. [PubMed]
- Dobrilovic N, Bennett S, Smith C, Edwards J, Luchette FA. Traumatic renal artery dissection identified with dynamic helical computed tomography. J Vasc Surg 2001;34:562-4. [Crossref] [PubMed]
- Santucci RA, Fisher MB. The literature increasingly supports expectant (conservative) management of renal trauma--a systematic review. J Trauma 2005;59:493-503. [Crossref] [PubMed]
- Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: is it worth doing in hemodynamically stable blunt trauma patients? Surgery 2010;148:695-700; discussion 700-1. [Crossref] [PubMed]
- Sangthong B, Demetriades D, Martin M, Salim A, Brown C, Inaba K, Rhee P, Chan L. Management and hospital outcomes of blunt renal artery injuries: analysis of 517 patients from the National Trauma Data Bank. J Am Coll Surg 2006;203:612-7. [Crossref] [PubMed]
- Brewer ME Jr, Strnad BT, Daley BJ, Currier RP, Klein FA, Mobley JD, Kim ED. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol 2009;181:1737-41. [Crossref] [PubMed]
- Breyer BN, McAninch JW, Elliott SP, Master VA. Minimally invasive endovascular techniques to treat acute renal hemorrhage. J Urol 2008;179:2248-52; discussion 2253. [Crossref] [PubMed]
- Bardin F, Chevallier O, Bertaut A, Delorme E, Moulin M, Pottecher P, Di Marco L, Gehin S, Mourey E, Cormier L, Mousson C, Midulla M, Loffroy R. Selective arterial embolization of symptomatic and asymptomatic renal angiomyolipomas: a retrospective study of safety, outcomes and tumor size reduction. Quant Imaging Med Surg 2017;7:8-23. [Crossref] [PubMed]
- Bent C, Iyngkaran T, Power N, Matson M, Hajdinjak T, Buchholz N, Fotheringham T.. Urological injuries following trauma. Clin Radiol 2008;63:1361-71. [Crossref] [PubMed]
- Toutouzas KG, Karaiskakis M, Kaminski A, Velmahos GC. Nonoperative management of blunt renal trauma: a prospective study. Am Surg 2002;68:1097-103. [PubMed]
- Chow SJ, Thompson KJ, Hartman JF, Wright ML. A 10-year review of blunt renal artery injuries at an urban level I trauma centre. Injury 2009;40:844-50. [Crossref] [PubMed]
- Hagiwara A, Sakaki S, Goto H, Takenega K, Fukushima H, Matuda H, Shimazaki S. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma 2001;51:526-31. [Crossref] [PubMed]