Diffuse glioblastoma resembling acute hemorrhagic leukoencephalitis
Introduction
The term “diffuse glioma” is currently used to describe as a special neoplastic pattern indicating a widespread brain invasion involving three or more cerebral lobes, frequent bilateral growth and possible extension to infratentorial structures, common to different glioma subtypes The term “gliomatosis”, formerly used in these cases, has been recently dismissed in the 2016 WHO classification (1,2). Here, we discuss the presentation and diagnosis of a diffuse glioblastoma in a young male who was initially suspected of having acute hemorrhagic leukoencephalitis (AHLE), a variant of acute disseminated encephalomyelitis (ADEM), and highlight the need of a synergic radiological and histological work-up to reach a correct diagnosis.
Case presentation
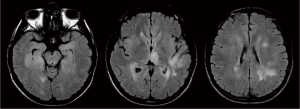
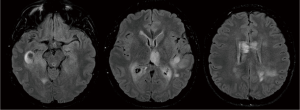
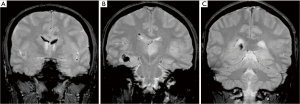
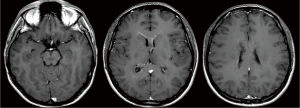
A previously healthy 24-year-old man presented with diplopia, concomitant with upper respiratory airway infection. He had no fever. The neurological examination showed right sixth cranial nerve paresis and dissociated nystagmus in the right lateral gaze. Brain MRI showed a hyperintense T2- and fluid-attenuated inversion recovery (FLAIR)-weighted (w) oval area in the left thalamus, associated with involvement of bilateral deep temporal (mostly on the right side), left posterior temporal, and bilateral peritrigonal and centrum semiovale white matter (Figure 1). Twenty days later, MRI showed a slight disease progression, with involvement of the corpus callosum (CC), and heterogeneously hypointense focal lesions on T2-, Gradient-Echo (GE) T2* and FLAIR-w images, suggestive of hemorrhagic components (Figures 2,3). Some of these lesions showed restricted diffusion, particularly in the right peritrigonal region and in the left centrum semiovale. After contrast medium injection, mild enhancement of the periventricular ependyma and CC was observed (Figure 4), with marked increase in the relative cerebral blood volume (rCBV) (Figure 5). The hematochemical and microbiological tests of cerebrospinal fluid (CSF), including screening for JCV and HIV, were normal, and oligoclonal bands were absent. The spinal MRI and visual evoked potentials were also normal. Additional tests on serum (celiac disease screening, neoplastic markers, autoimmune profile) and total-body CT scan were negative. The diagnosis of acute hemorrhagic leukoencephalitis (AHLE), an ADEM variant, was presumed and a treatment with high dose intravenous dexamethasone was started, but soon stopped due to intolerance and lack of clinical benefit. In the next few days the patient presented progressive clinical worsening with gait instability, ataxia, ophthalmoplegia, epilepsy and symptoms of intracranial hypertension. An open brain biopsy was thus performed in the area with the highest CBV value (right peritrigonal region, Figure 5, yellow arrows) and the histological diagnosis was glioblastoma (Figure 6). In view of the patient’s clinical status, imaging data, histological diagnosis, and lack of indication for radiotherapy, chemotherapy was started (Temozolomide 150 mg/m2 qDay for 5 days, repeated at 28-day cycles). He remained clinically stable for one month, then rapidly deteriorated (Glasgow Coma Scale score: 6). Brain MRI showed severe progression of the disease with subependymal dissemination. For this reason, Temozolomide was stopped and palliative care was started. The patient died four months after the onset of neurological disease.
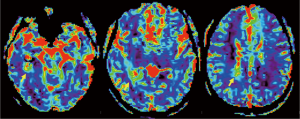
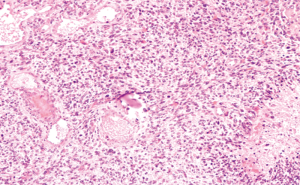
Discussion
This case initially showed several elements evocative of the rare and severe hemorrhagic variant of ADEM, which is an acute inflammatory demyelinating disease of the CNS (3). In fact, age at onset, concomitant airway infection, clinical presentation, and some features of the two initial brain MRIs (multifocal gray and white matter lesions associated with focal hemorrhage) were all suggestive of AHLE. The differential diagnosis included neoplastic disease, either lymphomatosis cerebri (LC) or diffuse glioma. LC is a rare variant of primary CNS lymphoma, characterized on MRI by a diffuse infiltration of the cerebral white matter, with variable contrast enhancement at the early stage and frequent extension of the lesions to the brainstem, thalamus, and basal ganglia (4). LC typically shows a good response to pulse steroid therapy. This diagnosis was initially hypothesized but later excluded during the clinical and radiological follow-up. Diffuse glioblastoma is a rare condition, often presenting with a variety of signs and symptoms that may mimic other diseases. The involvement of at least three cerebral lobes by an infiltrative tissue, the lack of a single discrete mass and pathological confirmation of diffuse glioma (WHO grade IV) indicate the diagnosis. However, the imaging features may also be present in CNS inflammatory diseases, vasculitis, encephalitis, leukoencephalopathies, especially when there is no obvious mass effect (2,5-10). While cases of demyelinating diseases mimicking gliomas are well represented in the literature (11,12), the opposite is a rather rare occurrence, mainly reported in the pediatric (13-15) or young (16) age. Thus, clinical and radiological features should be the first data directing the treating physician, as histological confirmation obtained through biopsy may provide a partial picture of the tumor (15). Diffuse glioma presents with diffuse, poorly-defined hyperintense lesions on T2-w and FLAIR images, and with variable contrast-enhancement, making imaging studies sometimes rather nonspecific; in these cases, the integration of functional (spectroscopy, perfusion) and morphological information is helpful in guiding the bioptic procedure (17). Diffuse expansion of the CC, mass effect, and progressive deterioration during follow-up may be considered characteristic. Therefore, we emphasize the need to maintain diffuse glioma in the differential diagnosis of patients with an encephalitis-type presentation; possibly considering the need of biopsy in either clinical or neuroradiologic equivocal cases.
Diffuse glioma usually predominates in the white matter: interestingly, a major involvement of the gray matter is associated to increased response to chemotherapy (18). However, the prognosis is poor, with somewhat better outcome associated with a low grade histology, young age, and good performance status (19). No consensus on the treatment is available at present, so that therapeutic choices should be tailored to each individual case (20).
Conclusions
In conclusion, this case highlights the diagnostic difficulties of diffuse gliomas when clinical and radiological features are not specific. Diffuse gliomas and AHLE are both rare and severe entities, but the differential diagnosis is very important because the use of various combinations of immunosuppressive agents in the latter (corticosteroids, immunoglobulin, plasmapheresis and cyclofosfamide) proved beneficial in some cases. MRI can establish the diagnosis in an appropriate clinical setting, but a histological definition may be necessary to confirm the diagnosis, possibly guided by functional information.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: We confirm that the patient had given a written consent to perform imaging studies and to all treatment modalities, which is archived in his clinical records.
References
- Herrlinger U, Jones DT, Glas M, Hattingen E, Gramatzki D, Stuplich M, Felsberg J, Bähr O, Gielen GH, Simon M, Wiewrodt D, Schabet M, Hovestadt V, Capper D, Lichter P, Pfister SM, Weller M, Reifenberger G. Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 2016;131:309-19. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Wender M. Acute disseminated encephalomyelitis (ADEM). J Neuroimmunol 2011;231:92-9. [Crossref] [PubMed]
- Kitai R, Hashimoto N, Yamate K, Ikawa M, Yoneda M, Nakajima T, Arishima H, Takeuchi H, Sato K, Kikuta K. Lymphomatosis cerebri: clinical characteristics, neuroimaging, and pathological findings. Brain Tumor Pathol 2012;29:47-53. [Crossref] [PubMed]
- Rajz GG, Nass D, Talianski E, Pfeffer R, Spiegelmann R, Cohen ZR. Presentation patterns and outcome of gliomatosis cerebri. Oncol Lett 2012;3:209-13. [PubMed]
- Muccio CF, Di Blasi A, Esposito G, Brunese L, D'Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol 2013;78:66-9. [Crossref] [PubMed]
- Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L. Errors in neuroradiology. Radiol Med 2015;120:795-801. [Crossref] [PubMed]
- Battipaglia G, Avilia S, Morelli E, Caranci F, Perna F, Camera A. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML). Ann Hematol 2012;91:1327-8. [Crossref] [PubMed]
- Bucciero A, De Caro ML, Tedeschi E, De Stefano V, Bianco M, Soricelli A, Vizioli L, Cerillo A. Atypical pleomorphic xanthoastrocytoma. J Neurosurg Sci 1998;42:153-7. [PubMed]
- Tedeschi E, Soricelli A, Brunetti A, Romano M, Bucciero A, Iaconetta G, Alfieri A, Postiglione A, Salvatore M. Different thallium-201 single-photon emission tomographic patterns in benign and aggressive meningiomas. Eur J Nucl Med 1996;23:1478-84. [Crossref] [PubMed]
- Miller DH, Scaravilli F, Thomas DC, Harvey P, Hirsch NP. Acute disseminated encephalomyelitis presenting as a solitary brainstem mass. J Neurol Neurosurg Psychiatry 1993;56:920-2. [Crossref] [PubMed]
- Pakos EE, Tsekeris PG, Chatzidimou K, Goussia AC, Markoula S, Argyropoulou MI, Pitouli EG, Konitsiotis S. Astrocytoma-like multiple sclerosis. Clin Neurol Neurosurg 2005;107:152-7. [Crossref] [PubMed]
- Richard HT, Harrison JF, Abel TW, Maertens P, Martino AM, Sosnowski JS. Pediatric gliomatosis cerebri mimicking acute disseminated encephalomyelitis. Pediatrics 2010;126:e479-82. [Crossref] [PubMed]
- Zhao J, Bao X, Fu N, Ye J, Li T, Yuan Y, Zhang C, Zhang Y, Zhang Y, Qin J, Wu X. Disseminated encephalomyelitis-like central nervous system neoplasm in childhood. J Child Neurol 2014;29:NP28-34. [Crossref] [PubMed]
- Senatus PB, McClelland S 3rd, Tanji K, Khandji A, Huang J, Feldstein N. The transformation of pediatric gliomatosis cerebri to cerebellar glioblastoma multiforme presenting as supra- and infratentorial acute disseminated encephalomyelitis. Case report. J Neurosurg 2005;102:72-77. [PubMed]
- Lancellotti CL, Amary MF, Barbastefano AM, Tilbery CP. “Gliomatosis cerebri" simulating an acute diffuse encephalomyelitis. Case report. Arq Neuropsiquiatr 1997;55:488-95. [Crossref] [PubMed]
- Quarantelli M, Alfano B, Larobina M, Tedeschi E, Brunetti A, Covelli EM, Ciarmiello A, Mainolfi C, Salvatore M. Frequency encoding for simultaneous display of multimodality images. J Nucl Med 1999;40:442-7. [PubMed]
- Kaloshi G, Guillevin R, Martin-Duverneuil N, Laigle-Donadey F, Psimaras D, Marie Y, Mokhtari K, Hoang-Xuan K, Delattre JY, Sanson M. Gray matter involvement predicts chemosensitivity and prognosis in gliomatosis cerebri. Neurology 2009;73:445-9. [Crossref] [PubMed]
- Kaloshi G, Everhard S, Laigle-Donadey F, Marie Y, Navarro S, Mokhtari K, Idbaih A, Ducray F, Thillet J, Hoang-Xuan K, Delattre JY, Sanson M. Genetic markers predictive of chemosensitivity and outcome in gliomatosis cerebri. Neurology 2008;70:590-5. [Crossref] [PubMed]
- Rudà R, Bertero L, Sanson M. Gliomatosis cerebri: A review. Curr Treat Options Neurol 2014;16:273. [Crossref] [PubMed]


