Paracoccidioidomycosis: level of pulmonary sequelae in high resolution computed tomography images from patients of two endemic regions of Brazil
Introduction
Paracoccidioidomycosis (PCM) is a systemic mycosis geographically restricted to Central and South America (1-4) and is endemic to populations who live in rural areas (1,5,6). The majority of the PCM cases (around 80%) are reported in Brazil (7,8), which is the first one cause of death among systemic mycoses disease in the country (1,9). Different distribution in endemic areas and the difficult to notify the disease are facts, which contributes negatively to establish incidence and prevalence of PCM (10,11).
PCM can be caused by fungi of the Paracoccidioides genus, comprising P. brasiliensis complex, consisting of four cryptic species (S1, PS2, PS3 and PS4) (2,3,12) and P. lutzii. (initially Pb01, a new clade) (3,6). The Paracoccidioides genus exhibits extensive genetic variability, as well as a possible correlation between genotype distribution and geographical isolation, culture adaptation, drug resistance and virulence (6,13,14).
All of the species of Paracoccidioides that have been identified to date are causal agents of PCM (2). However, the presence of the disease is associate to mild temperatures, fertile soils and high humidity, which makes its distribution not homogeneous (15). Several unexplained regional peculiarities have been noted in the diagnosis, clinical manifestations, and treatment of the disease (2). These regional peculiarities urgently need further exploration to rapidly and specifically detect Paracoccidioides species that are involved in the disease and improve diagnostic and therapeutic methods, thus reducing the sequelae of the disease (2). Many studies have explored the evolutionary mechanisms that were responsible for the current geographic distribution of phylogenetic species (S1, PS2, PS3, PS4, and P. lutzii) (2). However, these cryptic species still require further studies to correlate their ecoepidemiology, diagnosis, clinical manifestation and response to antifungal treatments (12).
Pulmonary PCM causes sequelae that can persist, even after effective treatment, causing destruction of the lung parenchyma which may be substituted by areas of fibrosis, emphysema, or a combination of both (16). Pulmonary alterations caused by PCM are evaluated preferable with high-resolution computed tomography (HRCT) since it is an image modality with additional information about the morphological characteristics and distribution of pulmonary lesions (17-19). Thus, it is extremely important to quantify the sequelae that are caused by PCM to better understand the mechanisms of the disease, to find possible variations between different forms of the disease and to allow the development of better therapies, according to the characteristics of the disease.
The purpose of the present study was to evaluate the level of pulmonary sequelae caused by PCM from two endemic regions of Brazil (Botucatu, SP, in the southeast region and Campo Grande, MS, in the west central region). Fibrosis and emphysema were objectively quantified in treated patients with the chronic form of PCM. The quantifications were performed according to an established and validated computational method, using HRCT exams. The results were compared with clinical data.
Methods
Ethics statement
The present study was developed with approval from the ethics committee of the authors’ institutions and national review panels under protocol no. 3883-2011. For the study, all patient medical data were anonymized.
Patient selection
We performed a retrospective analysis of HRCT exams from 32 treated patients with the chronic form of PCM between 2010 and 2015. The Botucatu group included 17 patients (17 men; age range, 36–67 years) with PCM at admission to the Infectious and Parasitological Diseases Service of the Medical School Hospital of Botucatu, Universidade Estadual Paulista, Botucatu, SP. The Campo Grande group included 15 patients (15 men; age range, 40–76 years) with PCM at admission to the Maria Aparecida Pedrossian Medical School Hospital, Campo Grande, MS. Botucatu and Campo Grande are important endemic regions which accounts with reference hospitals for the PCM treatment.
PCM was confirmed by the identification of typical P. brasiliensis yeast forms. It was detected by a positive finding of specific serum antibodies by a double agar gel immune diffusion test (DID), together with radiological findings that suggested pulmonary involvement. Respiratory complaints and HRCT revealed interstitial and/or alveolar lesions, indicating a chronic character of the disease.
Patients were eligible for inclusion in the study if treatment with an anti-P. brasiliensis compound was successful (reflected by a negative serum anti-P. brasiliensis antibody result) (20) and if the chest HRCT revealed fibrotic scars and different amounts of emphysema. The period of treatment was 44.3±21.5 months, ranging between 7 to 94 months. Most part of patients (93.8%) were treated exclusively using trimethoprim-sulfamethoxazole commonly known as otrimoxazole (CMX), which has good results to treat PCM (20) and is distributed free of cost by Brazilian government. Patients were treated until the clinical cure was achieved, e.g., disappearance of the signs and symptoms exhibited by the patients at recruitment, other than those possibly associated with sequelae. Duration of symptoms was defined in this study as the period of time in which the patient had any associated PCM symptoms. The apparent cure was associated with clinical and serologic cure for more than 2 years after treatment discontinuance (20). Only patients who lived the entire life in one endemic region were selected, in order to avoid bias evaluation.
Patients were ineligible for the study if they presented unsuccessful treatment or another systemic or pulmonary disease of any cause (e.g., infectious, inflammatory, or neoplastic). Patients with any other historic of lung diseases were ineligible so that fibrosis were only due to PCM. Also, patients treated exclusively with any other medicament, such as itraconazol, were excluded from this work. Importantly, all of the patients were alcoholics and smokers.
The classification of clinical forms and severity was described by Mendes (20,21).
Data acquisition
Images were obtained as retrospective HRCT scans on a helical computed tomography (CT) scanner [SCT-7000TS, Shimadzu (Botucatu group); Aquileon, Toshiba (Campo Grande group)]. Axial sections (1-mm thickness) were obtained at 10-mm intervals throughout the entire chest, with 20–30 slices acquired for each patient. No contrast agents were administered for acquisition. An available set of 32 HRCT examinations of the patients’ lungs was scanned, 17 from Botucatu group and 15 from Campo Grande group. For each examination, the voxel distribution in Hounsfield units (HU) was obtained. In this study, assessed HRCT exams were acquired after achieving persistently negative serologic tests.
Clinical data were obtained from the medical records of each patient. The obtained data were age, sex, disease severity, symptom duration, clinical and apparent cure times, titers on double immunodiffusion (DID) test, and smoking history. These data were used for comparisons with quantifications of lung sequelae that were based on a computed algorithm (17).
Computed algorithm
The algorithm implemented herein objectively quantifies fibrosis and emphysema using methodology described and validated in literature (17). The algorithm was developed in Matlab R2013a (Mathworks, Natick, MA, USA) and is based on histogram from lung tissues in HRCT exams. Validation of this method was made applying the algorithm in a virtual phantom with known amount of emphysema and fibrotic tissues (17).
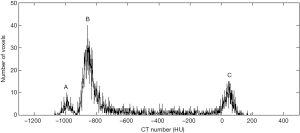
Steps of algorithm are briefly described hereafter. The typical histogram of lung images (Figure 1) presents three well-separated characteristic peaks of the different tissues: around −800 HU for normal tissue, −950 HU for emphysemic tissue, and 70 HU for fibrotic tissue.
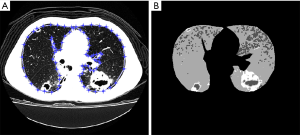
The lung was first manually segmented in each CT slice of the examination (Figure 2A). The segmented lung was then thresholded by analyzing the slice histogram. To quantify regions of sequelae in the thresholded images, an opening operation was applied to remove small-sized areas that likely resulted from density fluctuations rather than lung abnormalities. This resulted in a segmented image with four levels (outside lung areas, normal lung, emphysema, and fibrosis; Figure 2B). Final quantification was performed by determining the regions of the differentially labeled pixels (i.e., the classified lung volumes). The total quantity of each tissue was calculated applying these steps in all slices of the HRCT exam (17).
Statistical analyses
The statistical analyses were performed using Minitab Statistical Software (MINITAB Inc., State College, PA, USA) version 15. The results of the objective quantification of lung tissues and clinical data were compared between both groups of patients (Botucatu and Campo Grande). For the clinical data evaluation, independent differences between groups were analyzed using Student’s t-test or the Mann-Whitney U test for parametric and nonparametric data, respectively. χ2 tests were used for frequency comparisons of patients with severe-stage disease.
The lung tissue quantification was evaluated using analysis of covariance (ANCOVA). Before performing the ANCOVAs, all of the data were normalized. The ANCOVA was performed for each lung tissues quantification (fibrosis and emphysema) comparing Campo Grande e Botucatu group. Lung tissue quantifications were used as dependent variables and clinical data as the covariate. This analysis was performed since the two groups had significantly different clinical and apparent cure times and disease severity variables, which may have contributed to differences in lung sequelae. Therefore, these variables were corrected in the analysis, since they were used as covariates in an ANCOVA. Then, Tukey’s post hoc test was used to indicate significant differences between groups. Values of P<0.05 were considered statistically significant.
Results
The clinical and epidemiological data for the patients from Botucatu and Campo Grande groups are shown in Table 1. All of the patients were smokers. The antifungal drug used by the patients (93.8%) was exclusively CMX. The remaining 6.2% used CMX for the majority of treatment time, however received another drug at some period of treatment.
Full table
Results from evaluation of HRCT by radiologists showed that radiological findings, in PCM patients assessed in this study, were mainly fibrosis and emphysema, which are the most relevant findings for the clinic. HRCT findings such as cavitary nodules and ground-glass and tree-in-bud opacities are not evaluated, since they are prominent in pretreatment stage (17) and we included only patients successfully treated.
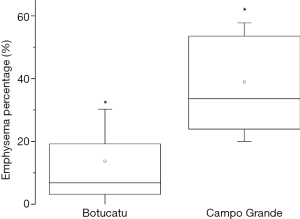
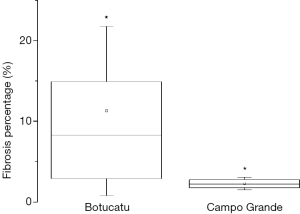
As a result of treatment, patients from Botucatu presented higher values of fibrosis in lungs, with significant differences between groups, as shown in Figure 3. On the other hand, patients from Campo Grande presented more emphysema. Statistical analysis revealed significant differences between groups, as shown in Figure 4. Differences found between groups are not due to variations in clinical data of the patients, since these data were used as covariates in ANCOVA analysis. Therefore, the differences found were corrected for clinical data distribution.

Discussion and Conclusions
We analyzed PCM patients from two different endemic Brazilian regions (Botucatu, SP, in the southeastern region and Campo Grande, MS, in the west central region), which are distant around 1,000 kilometers. This analysis was based on clinical data that were associated with objective quantifications of pulmonary sequelae from HRCT exams. These objective quantifications were performed by an established and validated computational method (17).
The two groups had similar characteristics with regard to age, symptom duration, smoking history, and titers on double immunodiffusion tests. However, the χ2 test results revealed differences in disease severity between groups. This test found more severe cases in the Campo Grande group, which may explain the greater clinical and apparent cure times in this group compared with the Botucatu group. Results of severity made by spirometry in PCM patients from Botucatu revealed that obstructive ventilator impairment (OVI) in 39% of patients were classified as mild, 38% as moderate and 23% as severe. For Campo Grande patients, 56% were classified as mild, 33% as moderate and 11% as severe. It is important to note that 23% and 40% from Botucatu and Campo Grande patients respectively, did not realize this exam. Although spirometry provides clinically relevant parameters of the functional status of the patient (22), spirometric results should be made using the same kind of instrument and testing procedure (23). Furthermore, the test is dependent on the patients’ cooperation (24). Therefore, even with a greater percentage of patients from Botucatu with severe spirometric results, it is not possible to infer that Campo Grande has less severity cases, since 40% of patients has not realized the exam.
Figure 3 shows that the presence of fibrosis was significantly higher in patients in the Botucatu group. The percentage of lungs that were affected by emphysema was significantly higher in the Campo Grande group (Figure 4).
The Campo Grande group had a significantly higher percentage of emphysema, which might be attributable to PCM, tobacco, or other factors. Smoke habit in PCM patients is almost universal and, thus, is not feasible to study emphysema in no smoker PCM individuals (25). Therefore, we cannot provide a definitive reason for the higher percentage of emphysema in Campo Grande group. However, we can associate the presence of fibrosis exclusively to PCM because the patients had no other systemic or pulmonary disease of any cause. The Campo Grande group had more severe cases, and we expected that this group would have a higher percentage of fibrosis. However, the present findings show that the Botucatu group had a higher percentage of fibrosis compared with the Campo Grande group. Since the significant differences of the clinical data variables presented between groups (disease severity, clinical and apparent cure times) were corrected by using ANCOVAs, this increased amount of fibrosis in Botucatu group may be associated with differences in the cryptic species of the two regions. Whereas the amount of fibrosis can vary due to some factors, such as period of treatment, differences in cryptic species, etc. more studies should be done to clarify the differences observed herein. As this was a retrospective study, information’s about biochemical and genotyping tests was not available, given that PCM patients’ lives in rural area with limited resources. However, the developed methodology was applied with success to assess differences in amount of sequelae between groups.
These findings cannot be explained just with clinical aspects evaluated in this study. However, to date, there are different geographic distributions of Paracoccidioides genus. Theodoro et al. identified predominance of cryptic species S1 and PS2 of complex P. brasiliensis in Botucatu isolates whereas P. lutzii prevailed in the central west region of Brazil (26). In addition, other differences have been observed among Paracoccidioides species. Teixeira et al found morphologic variations between conidia of complex P. brasiliensis and P. lutzii (27). Studies evaluating double immunodiffusion (DID) test with sera from patients infected by different cryptic species complex P. brasiliensis and P. lutzii revealed wide variation in reactivity, suggesting significant differences among these fungi (12,28,29). In 2016, Siqueira et al. found distinct patterns of host-parasite interaction and pathology caused by P. brasiliensis and P. lutzii (10).
Prevention of human exposure to P. brasiliensis may be achieved if their survival and growth conditions are known, which can be possible by studying the geographical distribution of PCM (30). Studies have reported only the morphology of the different species, and the different levels of compromise that each species causes still need to be determined (31). However, inferring the biogeographic distribution of Paracoccidioides is not a simple matter due to long latency period of PCM, constant migration of human hosts and scarcity of environmental isolates (26).
Paracoccidioides genus is divided into five phylogenetic lineages, which may be a possible reason for the different clinical involvement. Further studies of immunopathogenesis are needed to better understand the possible associations between the different cryptic species and different clinical manifestations of PCM. Furthermore, more studies are necessary to geographically map these species in endemic regions. These studies may help the physicians better understand the mechanisms that underlie the variability in the outcomes of PCM patients, that could modulate the patient’s immune response resulting in less time of treatment which implies reduction of sequelae development (31). More studies also should be done to evaluate fibrosis using the proposed methodology herein in order to find a distinctive mark of residual PCM.
One of the limitations of our study was the limited sample size. This retrospective study used all data available in Botucatu and Campo Grande hospitals. PCM patients treated in both hospitals centers lives in the countryside and most of them have restricted financial resources. Therefore, the rate of patients who appropriately finishes the treatment is reduced. Furthermore, from all PCM patients, only a part of them realized HRCT exam, resulting in a restricted number of patients eligible in this study. A second limitation is that it was not possible to determine the subtype of PCM because biochemical and genotyping tests are not performed in clinical routine in both hospitals. Other limitation is that it was not possible to achieve a control group with smoking history patients, without any other pathology, to compare with emphysema results. In both hospitals, lung HRCT exams are only realized in patients who have systemic or pulmonary disease of any cause (e.g., infectious, inflammatory or neoplastic). Therefore, those patients were not eligible for the study herein.
In conclusion, the present study found significant differences between treated PCM patients infected by Paracoccidioides species from different geographical regions of Brazil. These differences were found in level of sequelae caused by PCM, mainly fibrosis, even after correction of clinical data, in order to allow comparison between groups. The findings presented herein may be due to different cryptic species of the Paracoccidioides genus from the analyzed geographic regions. The methodology presented may be useful to quantify sequelae in medical centers where the CT is the only exam available. Further, the presented tool has the potential to evaluate patients with different medication treatment, different disease severity and difference in causative agent. These findings may help unveil differences in terms of both morphology and the pulmonary consequences of this disease. These observations need to be explored further in an effort to rapidly and specifically detect Paracoccidioides species and improve diagnostic and therapeutic methods.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was developed with approval from the ethics committee of the authors’ institutions and national review panels under protocol no. 3883-2011. For the study, all patient medical data were anonymized.
References
- Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, Soares Felipe MS. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol 2013;8:1177-91. [Crossref] [PubMed]
- Teixeira MM, Theodoro RC, Nino-Vega G, Bagagli E, Felipe MS. Paracoccidioides species complex: ecology, phylogeny, sexual reproduction, and virulence. PLoS Pathog 2014;10:e1004397. [Crossref] [PubMed]
- Travassos LR, Taborda CP. New advances in the development of a vaccine against paracoccidioidomycosis. Front Microbiol 2012;3:212. [Crossref] [PubMed]
- Batista J Jr, De Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJF, Hahn RC. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses 2010;53:176-80. [Crossref] [PubMed]
- Franco M, Bagagli E, Scapolio S, da Silva Lacaz C. A critical analysis of isolation of Paracoccidioides brasiliensis from soil. Med Mycol 2000;38:185-91. [Crossref] [PubMed]
- Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MS. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol 2009;52:273-83. [Crossref] [PubMed]
- de Souza SP, Jorge VM, Xavier MO. Paracoccidioidomycosis in southern Rio Grande do Sul: a retrospective study of histopathologically diagnosed cases. Braz J Microbiol 2014;45:243-7. [Crossref] [PubMed]
- Vieira GD, Alves TD, de Lima SMD, Camargo LMA, de Sousa CM. Paracoccidioidomycosis in a western Brazilian Amazon State: Clinical-epidemiologic profi le and spatial distribution of the disease. Revista Da Sociedade Brasileira De Medicina Tropical 2014;47:63-8. [Crossref] [PubMed]
- Coutinho ZF, Silva D, Lazera M, Petri V, Oliveira RM, Sabroza PC, Wanke B. Paracoccidioidomycosis mortality in Brazil (1980-1995). Cad Saude Publica 2002;18:1441-54. [Crossref] [PubMed]
- Siqueira IM, Fraga CL, Amaral AC, Souza AC, Jeronimo MS, Correa JR, Magalhaes KG, Inacio CA, Ribeiro AM, Burguel PH, Felipe MS, Tavares AH, Bocca AL. Distinct patterns of yeast cell morphology and host responses induced by representative strains of Paracoccidioides brasiliensis (Pb18) and Paracoccidioides lutzii (Pb01). Med Mycol 2016;54:177-88. [Crossref] [PubMed]
- Paniago AM, Aguiar JI, Aguiar ES, da Cunha RV, Pereira GR, Londero AT, Wanke B. Paracoccidioidomycosis: a clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul. Rev Soc Bras Med Trop 2003;36:455-9. [Crossref] [PubMed]
- Machado GC, Moris DV, Arantes TD, Silva LR, Theodoro RC, Mendes RP, Vicentini AP, Bagagli E. Cryptic species of Paracoccidioides brasiliensis: impact on paracoccidioidomycosis immunodiagnosis. Mem Inst Oswaldo Cruz 2013;108:637-43. [Crossref] [PubMed]
- Desjardins CA, Champion MD, Holder JW, Muszewska A, Goldberg J, Bailao AM, Brigido MM, Ferreira ME, Garcia AM, Grynberg M, Gujja S, Heiman DI, Henn MR, Kodira CD, Leon-Narvaez H, Longo LV, Ma LJ, Malavazi I, Matsuo AL, Morais FV, Pereira M, Rodriguez-Brito S, Sakthikumar S, Salem-Izacc SM, Sykes SM, Teixeira MM, Vallejo MC, Walter ME, Yandava C, Young S, Zeng Q, Zucker J, Felipe MS, Goldman GH, Haas BJ, McEwen JG, Nino-Vega G, Puccia R, San-Blas G, Soares CM, Birren BW, Cuomo CA. Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genet 2011;7:e1002345. [Crossref] [PubMed]
- Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Nino-Vega G, Taylor JW. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol 2006;23:65-73. [Crossref] [PubMed]
- Barrozo LV, Mendes RP, Marques SA, Benard G, Silva ME, Bagagli E. Climate and acute/subacute paracoccidioidomycosis in a hyper-endemic area in Brazil. Int J Epidemiol 2009;38:1642-9. [Crossref] [PubMed]
- Tuder RM, el Ibrahim R, Godoy CE, De Brito T. Pathology of the human pulmonary paracoccidioidomycosis. Mycopathologia 1985;92:179-88. [Crossref] [PubMed]
- Alvarez M, Pina DR, de Oliveira M, Ribeiro SM, Mendes RP, Duarte SB, Miranda JR. Objective CT-based quantification of lung sequelae in treated patients with Paracoccidioidomycosis. Medicine 2014;93:e167. [Crossref] [PubMed]
- Giacomini G, Miranda JR, Pavan AL, Duarte SB, Ribeiro SM, Pereira PC, Alves AF, de Oliveira M, Pina DR. Quantification of Pulmonary Inflammatory Processes Using Chest Radiography: Tuberculosis as the Motivating Application. Medicine (Baltimore) 2015;94:e1044. [Crossref] [PubMed]
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131:4S-42S. [Crossref] [PubMed]
- Cavalcante RD, Sylvestre TF, Levorato AD, de Carvalho LR, Mendes RP. Comparison between Itraconazole and Cotrimoxazole in the Treatment of Paracoccidiodomycosis. Plos Neglected Tropical Diseases 2014.8. [PubMed]
- Mendes RP. Paracoccidiodomicose. In: Meira DA, editor. Terapêutica de Doenças Infecciosas e Parasitárias. Publicações Científicas Ltda; 1994.
- Behr J, Furst DE. Pulmonary function tests. Rheumatology (Oxford) 2008;47 Suppl 5:v65-7. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Leuppi JD, Miedinger D, Chhajed PN, Buess C, Schafroth S, Bucher HC, Tamm M. Quality of spirometry in primary care for case finding of airway obstruction in smokers. Respiration 2010;79:469-74. [Crossref] [PubMed]
- Costa AN, Benard G, Albuquerque AL, Fujita CL, Magri AS, Salge JM, Shikanai-Yasuda MA, Carvalho CR. The lung in paracoccidioidomycosis: new insights into old problems. Clinics (Sao Paulo) 2013;68:441-8. [Crossref] [PubMed]
- Theodoro RC, Teixeira MD, Felipe MSS, Paduan KD, Ribolla PM, San-Blas G, Bagagli E. Genus Paracoccidioides: Species Recognition and Biogeographic Aspects. PLoS One 2012.7. [PubMed]
- Teixeira Mde M, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol 2014;52:19-28. [PubMed]
- Queiroz Junior Lde P, de Camargo ZP, Tadano T, Rodrigues AM, Takarara DT, Gegembauer G, Araujo LM, Hahn RC. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from Central Western Brazil. Mycoses 2014;57:466-72. [Crossref] [PubMed]
- Gegembauer G, Araujo LM, Pereira EF, Rodrigues AM, Paniago AM, Hahn RC, de Camargo ZP. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis 2014;8:e2986. [Crossref] [PubMed]
- Barrozo LV, Benard G, Silva ME, Bagagli E, Marques SA, Mendes RP. First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl Trop Dis 2010;4:e643. [Crossref] [PubMed]
- Bernard G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia 2008;165:209-21. [Crossref] [PubMed]


