Dentinogenic ghost cell tumor—a rare case report with review of literature
Introduction
Dentinogenic ghost cell tumor (DGCT), also known as odontogenic ghost cell tumor, is considered as a rare neoplastic variant of calcifying odontogenic cyst (COC). COC constitute 1–2% of all odontogenic tumors and 2–14% of COC are mainly solid tumors (1).
In 1962, COC was recognized as a distinct clinicopathological entity by Gorlin et al. In 1971, WHO included it under classification of histological typing of odontogenic tumor, jaw cyst and allied lesions (2). In 1981, Praetorious et al. classified COC into cystic and solid neoplastic type and the term DGCT has been proposed for the neoplastic type (3). In 1983, Shear used the term dentinoameloblastoma for it due to resemblance of the features to the ameloblastoma and dentinoid production (4). In 2005, WHO included both type of COC under tumors, renamed COC as calcifying cystic odontogenic tumor (CCOT) and retained the term DGCT for the neoplastic type (5).
WHO [2005] defined DGCT as a locally aggressive tumor that is histologically characterized by strands and islands of ameloblastoma like epithelial cells infiltrating into mature connective tissue. Lesion also consists of aberrant keratinization in form of ghost cells with some of ghost cells undergoing calcification and production of variable amounts of dysplastic dentin (6). DGCT are of two types, extraosseous (peripheral) and intraosseous (central) type. Intraosseous DGCT are more aggressive, have an infiltrative growth pattern and a high recurrence rate after resection. Therefore intraosseous DGCT should be treated by extensive surgical resection with an adequate safety margin as compared to extraosseous variant (3,5).
The aim of this case report is to present an extremely rare case of intraosseous DGCT located in a right mandibular premolar-molar region in a 14-year-old male patient. Another important aspect is to make dentists familiar with this rare entity thus preventing it’s under diagnosis. The clinical, radiological and histopathological features of the lesion will be discussed along with a review of the existing literature.
Case presentation
A 14-year-old male patient reported to the department of oral medicine and radiology with complaint of swelling in the lower right premolar and molar region since 4 months (Figure 1). As reported by the patient swelling was slowly progressive but remained unchanged in size since 3 months. There was no history of pain associated with the swelling but the patient gave history of mobile teeth in the same region since 5 to 6 months. Medical, dental and family history was not relevant. All the vital and peripheral signs were within normal limits.
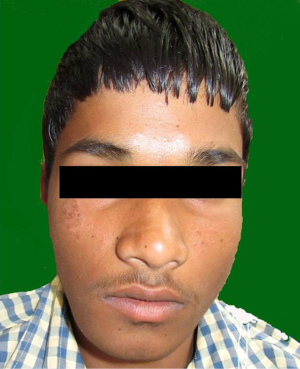
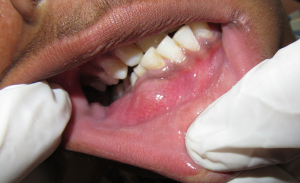
Extraoral examination revealed a solitary diffuse swelling in the lower right mandibular body measuring 2 cm × 1 cm approximately extending from right corner of the mouth till 2 cm from right ear and supero-inferiorly from line joining corner of mouth and tragus of ear till inferior border of mandible (Figure 1). On Physical examination bilaterally submandibular lymphadenopathy was present. Intraoral examination revealed a solitary swelling of size approximately 4 cm × 3 cm arising from buccal alveolar bone and extending from right mandibular central incisor to right mandibular second molar (Figure 2). On palpation swelling was mildly tender, bony hard with areas of softness. Missing teeth were permanent right mandibular canine and first premolar and retained deciduous right mandibular canine and first molar were noticed. Deciduous right mandibular canine and first molar were grade one mobile. On the basis of clinical examination provisional diagnosis of dentigerous cyst was given. Adenomatoid odontogenic tumor (AOT), ameloblastoma, calcifying epithelial odontogenic cyst were considered under differential diagnosis.
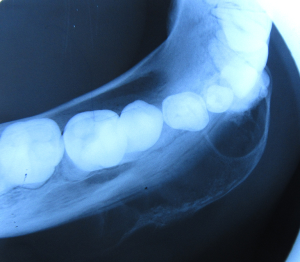
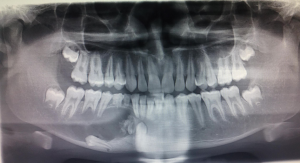
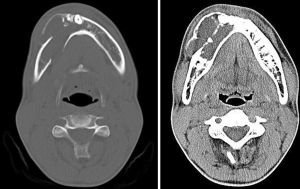
Patient was subjected to various radiographic investigations which included maxillary occlusal radiograph, orthopantomograph (OPG), and computed tomography (CT) scan. On occlusal radiograph radiolucent lesion with radiopaque calcified flecks was seen with thinning and expansion of buccal and lingual cortical plate and expansion of buccal cortical plate was more pronounced. Multiple compartments/locules were also noticed in the buccal cortex in the anterior part of the lesion (Figure 3). OPG revealed a predominantly radiolucent lesion with areas of opacification over the right mandibular body of size approximately 6 cm × 3 cm extending distally from right mandibular lateral incisor to mesially to right mandibular second molar, and inferiorly to lower border of mandible. Two unerupted teeth right mandibular canine and first premolar in continuation with perifollicular space, displacement of right mandibular first premolar inferiorly towards inferior border of mandible, vertical displacement of right mandibular canine to inferior border of mandible displacement of right mental foramen and inferior alveolar nerve was also noticed. In the anterior and superior portion of the lesion there were various dense calcified structures with heterogenous densities with resorption of root apex of right mandibular deciduous canine and first molar, and right mandibular second premolar and first molar (Figure 4). CT scan (axial view) revealed multiple locules/compartments predominantly in the buccal cortex with thinning of lingual cortex. Multiple discrete hyperdense calcified mass were noted in the anterior part of lesion (Figure 5). Radiographic differential diagnosis included calcifying epithelial odontogenic tumor (CEOT), AOT, DGCT.
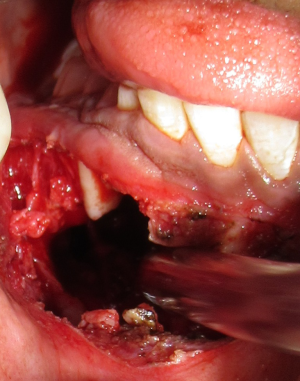
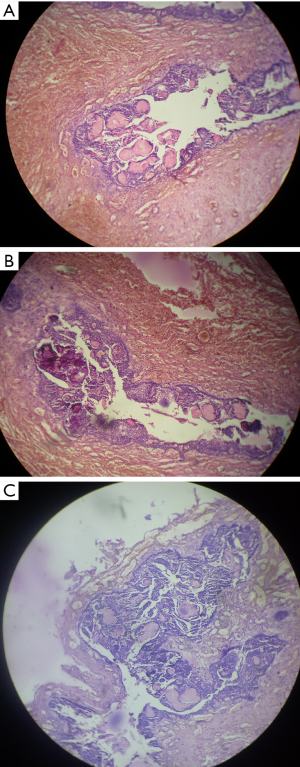
Followed by radiographic examination aspiration of fluid was done which was thin yellowish in colour, blood tinged and on cytological examination only red blood corpuscles (RBC) were found. All the haematological parameters were within the normal range. Patient consent was obtained prior to the surgery. Surgical enucleation of the lesion was done (Figure 6) and specimen was sent for histopathological examination. Histopathology revealed tumor comprised sheets and rounded islands of odontogenic epithelium with transformation of epithelial cells into ghost cells. Foci of dystrophic calcification and epithelial invagination were also seen (Figure 7). Based on Histopathologic findings, the present lesion was finally diagnosed as DGCT.
Discussion
COCs were first described by Gorlin and colleagues in 1962 as a separate entity of odontogenic origin. COCs account for 1–2% of all odontogenic tumors, in which 88.5% are cystic and the remaining 11.5% are solid tumors. As all lesions are not cystic, still it is debatable, whether COC is a cyst or a neoplasm. Based on this dualistic concept, WHO termed all cystic lesions as “CCOT” and the neoplastic entity as DGCTs (6). Intraosseous DGCT tend to be locally invasive, and patient age ranges from 12–75 years with a mean of 40 years (7,8), whereas extraosseous lesions exhibit limited growth potential and usually occurs in sixth decades, with an age range of 10–92 years (9). The lesions are common in males, involving any tooth-bearing area of the jaws, with cases about equally distributed between the mandible and maxilla (4,10). Intraosseous lesions mainly occur in the canine to first molar region, usually present as a painless bony swelling although some cases may present with slight numbness and pain (11-13). Extraosseous lesions exhibit predilection for anterior regions, and usually arise in the edentulous areas. They present as a firm, painless nodule on the gingival or alveolar mucosa. In the present case, the lesion was seen in a 14-year-old male patient in the right mandibular premolar molar region in comparatively younger age and presented as a painless swelling since 4 months.
Radiographic findings of the present case were consistent with the previous cases of DGCT in which it appeared radio graphically as a completely radiolucent lesion or mixed radiolucent-radiopaque lesion depending upon degree of calcification. Majority of cases are unilocular but multilocular lesions may be observed. These tumors are typically well defined, often expansile and may result in resorption and divergence of roots of adjacent teeth (2,14). In addition to the above findings, in the present case, displacement of right mandibular first premolar inferiorly towards inferior border of mandible, displacement of right mental foramen and inferior alveolar nerve and vertical displacement of right mandibular canine to inferior border of mandible was also seen radio graphically.
Odontogenic tumors mainly AOT and CEOT may resemble this rare entity. AOT is an uncommon tumor of odontogenic origin and unlikely DGCTs they occur commonly in females than males (2:1), mostly in the second decade and in the anterior aspect of the jaws mainly in the incisor, canine and premolar regions in which canine region is most common site (15). These tumors are usually in the dimensions of 1.5 to 3 cm, though tumors up to larger size, i.e., 12 cm size have also been reported. They are usually associated with missing teeth and present as painless swelling of jaw. Radiographically, they appear as a well circumscribed unilocular radiolucency and sometimes, faint radiopaque foci or clusters of ill-defined radiopacities are seen within the radiolucency, thus giving a flocculent pattern, which are better visualized on intraoral periapical radiographs than panoramic radiographs. But in AOT usually displacement of neighboring teeth due to tumor expansion is seen rather than root resorption which is a common finding in DGCTs (16).
CEOT is another benign odontogenic tumor which presents as a slow growing painless hard swelling in the premolar molar regions. These tumors more commonly occur in patients in 4th to 6th decades of life and occur primarily in the mandible, premolar molar area (mandible:maxilla ratio 2:1). Their association with an impacted tooth, most often first or second molar has also been reported (17). Radiographically, depending on the stages of development, CEOT may present variable radiographic appearances unilocular, multilocular, and nonloculated. Radiographs taken in the early stage of these tumors may reveal a completely radiolucent area around the crown of a mature, unerupted tooth. Later on multiple radiopacities of varying size may develop within the radiolucent area. In some cases small, thin, opaque trabeculae may cross the radiolucency in many directions, thus giving a multilocular or honeycomb pattern. CEOT have slightly lesser recurrence rate than intraosseous DGCT (17,18).
Both intraosseous and extraosseous variants of DGCTs exhibit similar histopathological features. Microscopic examination shows an unencapsulated, predominantly solid tumor with an infiltrative growth pattern. The tumor’s major components include ameloblastoma-like epithelial cells and ghost cells, often accompanied by dysplastic dentin. The epithelial cells with ameloblastic differentiation are arranged in sheets, islands, and nests within a background of mature fibrous connective tissue. Microcyst formation occasionally may be seen within the epithelial islands, with absence of mitotic activity in most cases (19). Scattered among the tumor epithelium are variable amounts of ghost cells. The ghost cells appear as eosinophilic epithelial cells which have lost their nuclei. A central void or “ghosted outline” is seen in place of a nucleus. These unusual cells are thought to result from aberrant keratinization or coagulative necrosis. The ghost cells often undergo calcification, which appear as fine basophilic granules or coarser basophilic masses. Production of dysplastic dentin or dentinoidal so may be seen in association with the tumor epithelium. The dysplastic dentin appears as amorphous masses of eosinophilic material. Occasionally, ghost cells may become entrapped by this dysplastic dentin (3,19). Histopathological findings were comparable to the current case characterized by ameloblastoma like epithelial cells infiltrating into connective tissue. In addition, peculiar “ghostcells” with dystrophic calcification were also noticed.
The epithelium in ameloblastoma and DGCT shows similarities. However, recognition of ghost cells, dysplastic dentin, and other calcifications typically makes it straightforward to distinguish the DGCT from ameloblastoma (9). It is important to distinguish the DGCT from its malignant counterpart odontogenic ghost cell carcinoma. Both lesions may exhibit ghost cells and infiltrative growth. However, characteristic microscopic features of odontogenic ghost cell carcinoma i.e., hypercellular proliferation of small cells with scanty cytoplasm, hyperchromatic nuclei, brisk mitotic activity, in some cases necrosis helps to distinguish it from DGCT. Thorough microscopic examination of DGCTs is advised, because malignant transformation into an odontogenic ghost cell carcinoma is possible (20). The CCOT is closely related to the DGCT, with both lesions exhibiting ameloblastoma-like epithelium, ghost cells, and dentinoid. However, CCOT is primarily cystic, whereas DGCT is primarily solid, although in some cases macrocystic components may be seen.
Early diagnosis of DGCT is essential for better prognosis of the patient and treatment plan usually differs for both variants of DGCT due to difference in recurrence rate and malignant potential. Intraosseous lesions generally require block excision or segmental resection with adequate safety margins, depending upon their size or anatomic extent and have high rate of local recurrence after limited local resection or following conservative therapy whereas extraosseous lesions are usually treated by conservative local excision. The recurrence potential of intraosseous lesions appears to be similar to that for conventional ameloblastoma. Recurrent cases usually occur 5–8 years following initial treatment. In contrast, no recurrences have been reported for extraosseous cases (3,20). Kasahara et al., reviewed 11 patients with intraosseous DGCT, and found that the recurrence rate was 36% (local recurrence occurred in 4 of the treated patients) (20). In another case by Sun et al., 7 patients with intraosseous DGCT were reviewed and 5 patients with conservative treatment showed recurrence of the lesion. In contrast, two patients had undergone aggressive surgical resection and no recurrence of the lesion was found (1). Malignant transformation of a DGCT into an odontogenic ghost cell carcinoma is rare (20). In our case the patient was treated by surgical enucleation and is under follow up. No recurrence of the tumor has been observed till date and the patient was asymptomatic.
Conclusions
In the present case study, a 14-year-old male patient was treated by surgical enucleation followed by curettage and is under follow up with no evidence of recurrence. Complete clinical, radiological and histopathological examination should be done to prevent the under diagnosis of this rare odontogenic tumor. Recurrence rate of intraosseous DGCT following resection remains one of the major concern, therefore regular follow-up of the patients is mandatory for better prognosis. Future recommends more clinical trials to determine best treatment modality for DGCT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Sun G, Huang X, Hu Q, Yang X, Tang E. The diagnosis and treatment of dentinogenic ghost cell tumor. Int J Oral Maxillofac Surg 2009;38:1179-83. [Crossref] [PubMed]
- Garcia BG, Ruiz Masera JJ, Zafra Camacho FM, Gutierrez CC. Intraosseous dentinogenic ghost cell tumor: Case report and treatment review. Rev Esp Cir Oral Maxilofac 2015;37:243-6. [Crossref]
- Praetorius F, Hjørting-Hansen E, Gorlin RJ, Vickers RA. Calcifying odontogenic cyst. Range, variations and neoplastic potential. Acta Odontol Scand 1981;39:227-40. [Crossref] [PubMed]
- Shear M. Calcifying odontogenic cyst. In: Shear M, editor. Cysts of the oral regions. 2nd ed. Bristol, UK: Wright, 1983:79-86.
- Li BB, Gao Y. Ghost cell odontogenic carcinoma transformed from a dentinogenic ghost cell tumor of maxilla after multiple recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:691-5. [Crossref] [PubMed]
- Singhaniya SB, Barpande SR, Bhavthankar JD. Dentinogenic ghost cell tumor. J Oral Maxillofac Pathol 2009;13:97-100. [Crossref] [PubMed]
- Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press, 2005:314.
- Juneja M, George J. Dentinogenic ghost cell tumor: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:e17-22. [Crossref] [PubMed]
- Praetorius F. Dentinogenic ghost cell tumour. In: Barnes L. editor. Surgical Pathology of the Head and Neck, Third Edition. CRC Press, 2009:1272-5.
- Ramaglia L, Esposito D, Bruno MP, Siano M. Dentinogenic ghost cell tumor: histopathology and clinical aspects in pediatric age. Minerva Stomatol 2012;61:509-17. [PubMed]
- Buchner A. The central (intraosseous) calcifying odontogenic cyst: an analysis of 215 cases. J Oral Maxillofac Surg 1991;49:330-9. [Crossref] [PubMed]
- Ledesma-Montes C, Gorlin RJ, Shear M, Prae Torius F, Mosqueda-Taylor A, Altini M, Unni K, Paes de Almeida O, Carlos-Bregni R, Romero de León E, Phillips V, Delgado-Azañero W, Meneses-García A. International collaborative study on ghost cell odontogenic tumours: calcifying cystic odontogenic tumour, dentinogenic ghost cell tumour and ghost cell odontogenic carcinoma. J Oral Pathol Med 2008;37:302-8. [Crossref] [PubMed]
- Candido GA, Viana KA, Watanabe S, Vencio EF. Peripheral dentinogenic ghost cell tumor: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e86-90. [Crossref] [PubMed]
- Konstantakis D, Kosyfaki P, Ebhardt H, Schmelzeisen R, Voss PJ. Intraosseous dentinogenic ghost cell tumor: a clinical report and literature update. J Craniomaxillofac Surg 2014;42:e305-11. [Crossref] [PubMed]
- Tegginamani AS, Kudva S, Shruthi DK, Karthik B. Adenomatoid odontogenic tumor - hamartoma/cyst or true neoplasm; a Bcl-2 immunohistochemical analysis. Indian J Dent Adv 2012;4:730-5.
- Patil NN, Nayyar AS, Wadhwan V. Adenomatoid odontogenic tumor: A series of four clinico-pathological variants. Int J Case Rep Imag 2014;(1):1-7.
- Cicconetti A, Tallarico M, Bartoli A, Ripari A, Maggiani F. Calcifying epithelial odontogenic (Pindborg) tumor. A clinical case. Minerva Stomatol 2004;53:379-87. [PubMed]
- Kamath G, Abraham R. Recurrent CEOT of the maxilla. Dent Res J (Isfahan) 2012;9:233-6. [Crossref] [PubMed]
- Bello IO, Qannam A, Al-Zahrani A, AlDosari A. Peripheral dentinogenic ghost cell tumor: report of a case and literature review. Int J Surg Pathol 2012;20:494-9. [Crossref] [PubMed]
- Kasahara K, Iizuka T, Kobayashi I, Totsuka Y, Kohgo T. A recurrent case of odontogenic ghost cell tumour of the mandible. Int J Oral Maxillofac Surg 2002;31:684-7. [Crossref] [PubMed]