
Heart-aorta-angle correlates left atrial low voltage areas formation in hypertensive atrial fibrillation patients
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia linked to a significantly higher risk of death and stroke (1). The pathophysiological mechanisms of AF have been extensively studied, including atrial fibrosis, inflammation and oxidative stress, and autonomic dysregulation (2). Hypertension is a significant risk factor for AF, and the risk of AF in hypertensive patients is 1.7 times higher than that in normotensive patients (3). Still, its potential mechanisms have not been fully elucidated.
Hypertension is associated with cardiac structural and electrical remodeling. Lau et al. found a more hypertrophic atrium, greater susceptibility to AF, and a notable reduction in atrial electro-conduction speed in hypertensive models (4). This electrical and structural remodeling developed earlier and over a broader range in the left atrium (LA) (5). Hypertension is also related to changes in external adjacent structures such as aortic dilatation, epicardial adipose tissue (EAT), thickness, and volume increase assessed by coronary computed tomography angiography (CCTA) or echocardiography (6,7). Previous studies have shown that LA fibrosis is more frequent in the area adjacent to the descending aorta. Extrinsic compression by descending aorta on the left atrium and pulmonary vein has been reported which can lead to pulmonary vein stenosis in the area adjacent to the descending aorta (8). However, the differences in close mechanical interaction between enlarged left atrium and dilated ascending aorta (AAo) in hypertensive AF patients compared to those without hypertension remain unexplored. Low voltage areas (LVAs) were usually considered a manifestation of LA remodeling. Therefore, our study aimed to analyse the relationship between LVAs in the atrial area adjacent to the AAo and heart-aorta-angle (HAA), and the subsequent recurrence after catheter ablation in AF patients with or without hypertension. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2025-80/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (No. KYLL-202411-024), and written informed consent was obtained from all participants. A total of 93 consecutive patients at Qilu Hospital of Shandong University from June 2022 to September 2023 who underwent CCTA for atrial thrombosis evaluation, and then received electro-anatomical mapping and catheter ablation for AF were included. The patients were divided into two groups according to with hypertension (HT) or normotension (NT). Hypertension was defined as confirmed office blood pressure of ≥140/90 mmHg three times on different days without using antihypertensive medication. The patient has a history of hypertension and is currently using antihypertensive drugs. Although the blood pressure is below the diagnostic threshold mentioned above, it should still be diagnosed as hypertension (9). CCTA examination was completed 2.1±1.1 days before the ablation procedure. Patients with previous AF ablation procedure or any ablation affecting the atrium, previous surgery on heart, esophagus or lungs, previous radiotherapy due to cancer in the thorax, severe valvular heart diseases, or other combined ablation procedures for supraventricular tachycardia, or ventricular arrhythmia were excluded. A detailed medical history including gender, age, smoking, AF type, previous diseases, use of antiarrhythmic drugs, laboratory test, and echocardiographic variables were collected from the database of the Qilu Hospital and provided by patients themselves at baseline. Figure 1 shows the flow of this study.
CCTA protocol and image analysis
All participants were scanned with a dual-source CT (Siemens SOMATOM Force). Scan parameters were as follows: collimation, 96×0.625 mm; gantry rotation time, 250 ms, temporal resolution, 66 ms. Reference tube voltage and current were set as 100 kV and 320 mAs (CARE kV and CARE dose 4D were used as reference). The timing of the image acquisition was determined by using a bolus tracking technique, with regions of interest (ROIs) placed in the AAo and trigger was set as 100 HU. Subsequently, a bolus of iodinated contrast agent (100 mL; Ultravist, 370 mg iodine/mL, Bayer) was injected at a rate of 4–5 mL/s, followed by a 40-mL saline flush using a dual-barrel power injector. Respiratory training was conducted before performing the CCTA scan. Each patient’s heart rate was maintained at less than 90 bpm to achieve better image quality, and β-blockers were administered if necessary. Retrospective electrocardiogram (ECG)-gated CCTA scanning was performed during breath-holding after inspiratory to minimize interference caused by respiratory motion. Images were reconstructed at the 75% phase of the R-R interval (10). Images were reconstructed with a 512×512 matrix, 0.5 mm slice thickness, and 0.5 increments. The acquired CCTA images were transferred to medical image analysis platform Timeslice (https://slice-doc.netlify.app/), ADAS 3D software (ADAS3D Medical, Barcelona, Spain), and 3D slicer (version 5.6.2) for measurement and further analysis. Heart-aorta-angle including ascending aorta-left atrium (AAo-LA) angle and AAo-left ventricular (AAo-LV) angle were measured to evaluate the relationship between LA and AAo according to relevant literatures. The AAo-LA angle was defined as the angle between the midline of AAo which means the line passing vertically through the center of aortic valve and midline of LA, that is, the line connecting the right pulmonary vein carina to the center of mitral valve; the AAo-LV angle means the angle between midline of AAo and left ventricular, that is, the line connecting the center of mitral valve and LV apex (Figure S1A,S1B) (11). Then, systematic assessment of aortic sinus dimensions including coronary cusp diameter, cusp-commissure distance, and sinus to sinus distance were made at the aortic root (Figure S1C-S1E) (12). LA volume was also calculated by the software. The close contact area (CoA) was defined as the region where the distance between the LA anterior wall (AW) and AAo was less than 1 mm.
All radiographic measurements were performed by one radiologist with 8 years of experience (G.F.) and one trained postgraduate studying for a degree in the cardiovascular field (Y.Y.).
Electro-anatomical mapping and ablation procedure
Antiarrhythmic medications were discontinued at least 5 half-lives before the procedure, and the electrophysiology study was conducted under conscious sedation. Intravenous heparin was used to maintain an activated clotting time greater than 300s during the LA mapping and ablation. LA geometry and a high-density bipolar voltage map with a minimum of 1,000 evenly distributed mapping points were created during sinus rhythm using a 3D-electroanatomical mapping system (CARTO 3; Biosense Webster), with a multispline catheter with 2-mm interelectrode spacing (PentaRay; Biosense Webster) and the CONFEDENSE software. Intracardiac echocardiography is used to re-construct AAo and LA model, guide transseptal puncture, monitor pericardial effusion, and observe the relationship between the aorta and the left atrium during ablation. For both paroxysmal and persistent atrial fibrillation (PeAF) patients, mapping procedure was done after circumstantial pulmonary vein isolation (PVI) and electrical conversion (when necessary, in most PeAF). Irrigated contact force sensing ablation catheters were used with minimum target contact force of >5 g. PVI involved wide antral circumferential ablation around pulmonary veins with the endpoint of electrical isolation. If extra pulmonary vein trigger foci, atrial tachycardia or flutter were induced, activation mapping was conducted to identify the tachycardia circuit and additional linear or focal ablation will be applied accordingly. In PeAF patients who could not restore sinus rhythm after PVI and electrical conversion, more extensive substrate modification was needed, then voltage mapping will be excluded from study for ablation factors.
LVAs definition and distribution
LVAs were identified as areas with a bipolar peak-to-peak voltage amplitude of less than 0.5 mV in sinus rhythm (13). We divided LA into five regions: septum, anterior, posterior, inferior, and lateral walls, omitting the left atrial appendage (LAA) (Figure S1F,S1G) (14). The mitral annulus and pulmonary veins beyond the antrum were excluded. Total and regional surface area, and LVAs area were recorded and used for further analysis. The distribution of LVAs was calculated by regional LVA in different walls/total LVA.
All electro-anatomical measurements were performed by a doctor with decades of experience in electrophysiology (T.C.) and one trained postgraduate (Y.Y.).
Follow-up
AF recurrence was defined as any documented fibrillation episode beyond a 3-month blanking period. A follow-up conducted at 3, 6, 9, 12 months after the procedure, by 12-lead ECGs and Holter monitoring. Patients were treated with antiarrhythmic drugs and anticoagulants for 3 months after ablation, and then withdrawal or maintenance according to AF recurrence or not. Most patients were followed-up at our outpatient clinic, and others were followed-up by telephone visit to collect ECG/Holter information.
Statistical analysis
Continuous variables were compared using either Student’s t-test or Wilcoxon rank-sum test and were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR). Categorical data are given as absolute values and percentages and were tested using the chi-square or Fisher exact test as appropriate. The correlation strength among the continuous variables was evaluated using the Spearman’s correlation coefficient. AF recurrence curves were constructed by using the Kaplan-Meier method. Cox regression analysis and receiver operating characteristic (ROC) curve were performed to determine the AAo-LA angle and AAo-LV angle cut-off values for prediction of AF recurrence. A two-tailed P value of <0.05 was considered statistically significant. Interobserver agreement was assessed by calculating intraclass correlation coefficient (ICC) in two-way mixed model for CCTA-based data and LVAs data. Statistical analyses were performed by were performed using R version 4.4.0 (R Foundation), along with Zstats 1.0 (www.zstats.net).
Results
Baseline characteristics
A total of 93 patients were included in our study. There were 48 patients (51.6%) with AF and concomitant hypertension. The clinical characteristics of the patients in per study group were shown in Table 1. A significant difference of stroke, CHA2DS2-VASc score, AAo diameter, interventricular septum (IVS) thickness, and left ventricular posterior wall (LVPW) thickness were observed between the two groups (all P<0.05). There were no significant intergroup differences in other clinical characteristics such as smoking history and other comorbidities.
Table 1
| Characteristics | Hypertensive group (n=48) | Normotensive group (n=45) | P value |
|---|---|---|---|
| Gender (male) | 29 (60.42) | 25 (55.56) | 0.635 |
| Age (years) | 66.00 (56.50, 71.00) | 63.00 (54.00, 69.00) | 0.191 |
| BMI (kg/m2) | 26.22±3.08 | 25.06±3.37 | 0.086 |
| Smoking | 19 (39.58) | 13 (28.89) | 0.278 |
| Paroxysmal AF | 25 (52.08) | 23 (51.11) | 0.925 |
| Duration of AF (months) | 16.00 (3.75, 51.00) | 13.00 (4.00, 37.00) | 0.972 |
| Coronary heart disease | 15 (31.25) | 8 (17.78) | 0.132 |
| Heart failure | 8 (16.67) | 7 (15.56) | 0.884 |
| OSAHS | 3 (6.25) | 0 (0.00) | 0.264 |
| Stroke | 8 (16.67) | 1 (2.22) | 0.045 |
| Diabetes mellitus | 13 (27.08) | 6 (13.33) | 0.100 |
| Antiarrhythmic drug use | 40 (83.33) | 35 (77.78) | 0.498 |
| CHA2DS2-VASc score | <0.001 | ||
| 0 | 0 (0.00) | 11 (24.44) | |
| 1 | 7 (14.58) | 19 (42.22) | |
| 2 | 12 (25.00) | 7 (15.56) | |
| 3 | 13 (27.08) | 5 (11.11) | |
| 4 | 9 (18.75) | 2 (4.44) | |
| 5 | 1 (2.08) | 1 (2.22) | |
| 6 | 3 (6.25) | 0 (0.00) | |
| 7 | 2 (4.17) | 0 (0.00) | |
| 8 | 1 (2.08) | 0 (0.00) | |
| NT-proBNP (pg/mL) | 504.85 (147.92, 1,123.50) | 433.50 (110.05, 881.95) | 0.280 |
| AAo (mm) | 32.79±3.17 | 30.62±3.50 | 0.002 |
| IVS (mm) | 11.00 (9.88, 12.00) | 10.00 (9.00, 11.00) | 0.001 |
| LVPW (mm) | 10.00 (9.00, 11.00) | 9.00 (9.00, 10.00) | 0.047 |
| LAD (mm) | 43.25±7.29 | 41.20±6.24 | 0.150 |
| LVDd (mm) | 47.00 (44.00, 51.00) | 47.00 (45.00, 50.00) | 0.923 |
| LVEF (%) | 62.00 (54.00, 66.00) | 61.00 (56.00, 65.00) | 0.799 |
Data are presented as mean ± standard deviation or median (1st quartile, 3rd quartile) or n (%). AAo, ascending aorta; AF, atrial fibrillation; BMI, body mass index; IVS, interventricular septum; LAD, left atrial diameter; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall; NT-proBNP, NT-pro B-type natriuretic peptide; OSAHS, obstructive sleep apnea-hypopnea syndrome.
AAo-LA features and LVAs distribution
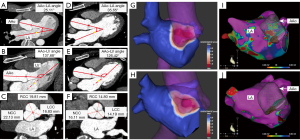
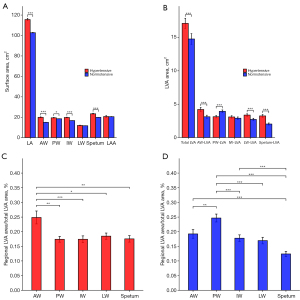
Anatomical features measured by CCTA were shown in Table 2. Dilation of AAo in HT group was shown by larger AAo, non-coronary cusp (NCC), right-coronary cusp (RCC) diameter, NCC-commissure, left-coronary cusp (LCC)-RCC distance, and LCC-NCC distance (all P<0.01). Compression of AAo on LA was shown by smaller AAo-LA angle, larger AAo-LV angle and CoA (all P<0.01). Representative CCTA images of HAA, aortic sinus diameter, CoA, and LA voltage mappings in HT and NT groups were shown in Figure 2A-2J. The total and individual regional surface area of LA were larger in HT group compared to NT group, except for lateral region and LAA (all P<0.05; Figure 3A). For LVAs features, larger total LVAs area were observed in HT group compared to NT group, especially in AW (P<0.001; Figure 3B). The proportion of LVA in AW was highest among different regions in HT group (AW-LVAs vs. other walls, all P<0.05; Figure 3C), but in NT group, the most proportion of LVAs was posterior wall (PW-LVAs vs. other walls, all P<0.01; Figure 3D). All ICC values for CCTA-based data and electro-anatomical data were greater than 0.9.
Table 2
| CCTA-based measurements | Hypertensive group (n=48) | Normotensive group (n=45) | P value |
|---|---|---|---|
| AAo-LA angle (°) | 29.11±2.87 | 31.83±2.04 | <0.001 |
| AAo-LV angle (°) | 132.22 (129.80, 134.59) | 129.33 (127.38, 131.87) | <0.001 |
| Diameter of AAo (mm) | 34.64 (32.17, 37.52) | 30.85 (29.84, 34.53) | 0.003 |
| LAD (mm) | 44.78±7.11 | 41.96±7.98 | 0.075 |
| LAV (mL) | 135.55 (116.67, 149.49) | 110.45 (95.34, 124.67) | <0.001 |
| CoA (cm2) | 5.69±2.11 | 4.49±2.07 | 0.006 |
| LCC (mm) | 19.81 (17.82, 20.20) | 18.61 (17.88, 19.23) | 0.122 |
| RCC (mm) | 18.98±1.08 | 17.85±1.53 | <0.001 |
| NCC (mm) | 20.80 (19.37, 21.71) | 19.11 (17.66, 19.69) | <0.001 |
| LCC-commissure distance (mm) | 34.46 (32.85, 35.96) | 34.10 (32.70, 34.94) | 0.305 |
| RCC-commissure distance (mm) | 33.74 (32.48, 35.02) | 32.56 (31.10, 34.84) | 0.056 |
| NCC-commissure distance (mm) | 34.85±2.57 | 33.54±2.14 | 0.009 |
| LCC-RCC distance (mm) | 32.77 (31.27, 34.57) | 31.43 (29.64, 33.48) | 0.007 |
| LCC-NCC distance (mm) | 31.91±1.86 | 30.54±2.33 | 0.002 |
| RCC-NCC distance (mm) | 31.68±1.97 | 31.04±1.66 | 0.096 |
Data are presented as mean ± standard deviation or median (1st quartile, 3rd quartile). AAo, ascending aorta; CCTA, coronary computed tomography angiography; CoA, contact area; LA, left atrium; LAD, left atrial diameter; LAV, left atrial volume; LCC, left coronary cusp; LV, left ventricle; NCC, noncoronary cusp; RCC, right coronary cusp.
Association between AAo-LA features and AW-LVAs
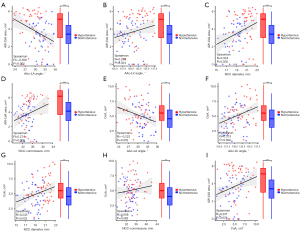
The above data showed that there were significant differences between HT and NT patients in statistical data such as HAA, NCC diameter, NCC-commissure distance, CoAs, and AW-LVAs. Therefore, the correlation between these factors was further analyzed. AAo-LA angle was correlated negatively with AW-LVA (r=−0.358, P<0.001; Figure 4A), additionally AAo-LV angle (r=0.233, P=0.024; Figure 4B), NCC diameter (r=0.324, P=0.002; Figure 4C), and NCC-commissure distance (r=0.274, P=0.008; Figure 4D) exhibited significant positive correlations with it. As for CoA, AAo-LA angle was also found a negative correlation with it (r=−0.222, P=0.032; Figure 4E). Both AAo-LV angle (r=0.313, P=0.002; Figure 4F) and NCC diameter (r=0.233, P=0.025; Figure 4G) were positively associated with CoA, but there was not an obvious relationship between NCC-commissure distance and CoA (P=0.583; Figure 4H). There was also a positive correlation between AW-LVA and CoA (r=0.231, P=0.026; Figure 4I).
Follow-up
During a follow-up of 12 months, AF recurred in 16 patients (33.3%) in hypertensive group and in 7 patients (15.6%) in normotensive group by Kaplan-Meier curves (P=0.041; Figure 5A). In univariate and multivariate Cox regression analysis, AAo-LA angle and AAo-LV angle showed predictive value for the recurrence (Table 3). The area under the ROC curve of AAo-LA angle and AAo-LV angle was 0.660 [95% confidence interval (CI): 0.538–0.781; P<0.01] and 0.754 (95% CI: 0.638–0.870; P<0.01) for prediction of AF recurrence, and a cut-off of 31.78° for AAo-LA angle and 131.99° for AAo-LV angle for AF recurrence (Figure 5B,5C).
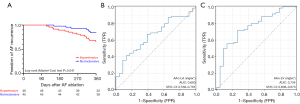
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Hypertension | 2.45 | 1.01–5.97 | 0.048 | – | – | – | |
| Male gender | 3.99 | 1.36–11.74 | 0.012 | – | – | – | |
| BMI | 1.12 | 0.98–1.27 | 0.088 | – | – | – | |
| AF duration | 1.01 | 1.01–1.01 | 0.016 | – | – | – | |
| Age | 0.96 | 0.92–0.99 | 0.035 | 0.96 | 0.93–0.99 | 0.037 | |
| CTNI | 1.02 | 1.01–1.04 | 0.001 | – | – | – | |
| LAD | 1.03 | 0.97–1.09 | 0.397 | – | – | – | |
| AAo | 1.05 | 0.93–1.17 | 0.431 | – | – | – | |
| AAo-LV angle | 1.29 | 1.13–1.48 | <0.001 | 1.19 | 1.04–1.37 | 0.013 | |
| AAo-LA angle | 0.87 | 0.76–0.99 | 0.040 | 0.84 | 0.71–0.99 | 0.043 | |
| NCC | 1.94 | 1.38–2.73 | <0.001 | 1.79 | 1.24–2.57 | 0.002 | |
| AW-LVA | 1.32 | 1.02–1.71 | 0.037 | – | – | – | |
AAo, ascending aorta; AF, atrial fibrillation; AW-LVA, anterior wall-low voltage area; BMI, body mass index; CI, confidence interval; CTNI, cardiac troponin I; LA, left atrium; LAD, left atrial diameter; LV, left ventricular; NCC, non-coronary cusp.
Discussion
Main findings
This study presents detailed information on the atrial substrate mapping and anatomical changes associated with hypertension in AF patients. The following changes were seen in our study:
- An increase of LVAs in LA AW in hypertensive AF patients;
- Differences of AAo-LA relationship associated with the distribution of LVAs in AF patients with hypertension;
- The close CoA between AAo and LA correlates with LA AW LVAs;
- A predictive value of HAA for AF recurrence.
Interaction among hypertension, ascending aortic disease and AF
Hypertension is the most prevalent modifiable risk factor associated with AF, acting as an independent predictor of AF incidence and recurrence. Approximately 90% of AF patients exhibit a history of hypertension in several randomized clinical trials (15).
Prior animal models have established a pathological link between hypertension and atrial structural and electrical remodeling. In chronic hypertensive ovine model, electrophysiological alterations including bi-atrial conduction slowing, reduced atrial wavelength, and structural changes such as atrial myocyte hypertrophy were observed (16). Previous study also revealed there was a reciprocal influence between arterial stiffness which was regarded as a complication of hypertension and AF, and they share commonalities in molecular and pathophysiological mechanisms (17). There were also several studies finding that chronic hypertension was associated with atrial conduction slowing, reduced LA voltage and increased AF inducibility (18,19). Increased LA size and impaired atrial pump function, as indicated by peak late-diastolic mitral annular velocity, could predict AF in hypertensive patients (20). LA strain parameters measured by echocardiogram were smaller in hypertensive AF patients and could be useful predictors of AF occurrence in hypertensive patients (21). In our study, enlarged left atrium and dilated AAo were observed in hypertensive group, which are both well-known secondary changes following hypertension. Analysis of CCTA features and voltage mapping data revealed a close relationship between peri-cardiac anatomy and LA electro-anatomic substrate, such as enlarged AAo-LA angle and increased LVAs in hypertensive AF patients. Additionally, the recurrence is more common in hypertensive group in our study, which is consistent with previous studies (22,23).
Aortic dilation and thoracic aortic aneurysm (TAA) are also common complications of hypertension. The risk of TAA is 1.24 times greater in patients with AF compared to those without AF. Conversely, patients with AA had a higher risk for AF occurrence than those without TAA (24). The Aortic Concomitant Thoracic and Abdominal Aneurysm (ACTA) study showed that in patients with abdominal aortic aneurysms, AF was an independent factor associated with aortic dilation and TAAs (25). Some common molecular mechanisms including transforming growth factor β1, matrix metalloproteinases and so on may play a role in the processes (26,27). In previous reports, obvious compressions of left atrium by extracardiac structures are often incidental findings detected by imaging tools. Maria Hugas et al. reported a case of a large thoraco-abdominal aneurysm compressing the left atrium and leading to functional mitral valve stenosis after total aortic arch repair (28), and shared similar characteristics with the deformation of left atrium observed in our CCTA images. So next, we intended to explore in hypertensive AF patients, whether AAo-LA relationship differs from normotensive AF patients.
The value of HAA in cardiovascular diseases
HAA which means the angle between heart and AAo has been studied in different cardiovascular diseases. In patients with hypertrophic cardiomyopathy, there was a smaller left ventricular-aortic root angle which could predict dynamic left ventricular outflow tract obstruction independently of basal septal thickness (29). In addition, HAA is also measured as a part of accurate measurement of aortic root structure for preoperative assessment in transcatheter aortic valve replacement (30). Kauhanen et al. found that a smaller HAA associated with AAo dilatation and affects the blood flow in the proximal AAo (31). Actually, HAA always referred to the angle between LV and AAo, and the angle between LA and AAo were not measured. Aorta-LA angle and aorta-LV angle were observed to correlate with low voltage areas in AF patients, which showed mechanical pressure exerted by extra-cardiac structures on the left atrium may contribute to the development of LVAs associated with AF. We also found the difference of AAo-LA angle and AAo-LV angle in hypertensive and normotensive AF patients, which had an apparent correlation with LVAs in AW of left atrium. We supposed it may be a mechanical mechanism by which hypertension affects the occurrence and recurrence of AF.
Formation, distribution, and significance of LVAs in AF patients
Structural remodeling including cellular hypertrophy, fibrosis and apoptosis was commonly observed in AF models and patients. The presence of fibrosis, which alters cellular coupling, is a potential contributor to atrial activation abnormalities that initiate and sustain reentrant arrhythmias such as AF. Currently, magnetic resonance imaging (MRI) is primarily used to assess atrial fibrosis levels. However, the image quality of cardiac MRI remains limited, particularly when imaging the thin left atrial wall in comparison to the left ventricular wall. A substantial number of studies are known to suggest a correlation between atrial fibrosis and low voltage areas, and there is a high correlation between scarred areas identified by late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) and LVAs identified by electro-anatomical calibrations (32,33).
LVA is recognized as a significant substrate of arrhythmogenicity. Atul Verma et al. found that pre-existent left atrial scarring in patients undergoing PVI for AF is a powerful, independent predictor of procedural failure (34). The prospective WAVE-MAP AF study also demonstrated a clear association between LVA and AF recurrence, low-voltage area (<0.5 mV) >28% of the left atrium in sinus rhythm and >72% in AF was associated with a higher risk of AF/atrial flutter/atrial tachycardia recurrence at 12 months (35). Previous studies implied a close relationship between hypertension and LVAs. Wang et al. found that the average LA voltages in hypertensive patients was significantly lower than in non-hypertensive patients, and the area of LVAs was also significantly larger in hypertensive patients (18). In the DR-FLASH scale which is for predicting LA LVAs, hypertension is included as an independent risk factor (36). Overall, these studies revealed a close relationship between hypertension, AF and LVA. Our research demonstrates that hypertensive AF patients have a smaller AAo-LA angle, which is associated with an increased LVA area in the AW of LA. The closer AAo-LA interaction relationship may increase the mechanical contact, which relates to LVAs formation in left atrium and contribute to the atrial fibrosis in hypertensive AF patients.
Previous studies showed the LVAs are frequently found in the anterior, posterior wall and septum in left atrium. Also, it was noticeable in normotensive patients, areas of LVA were larger than in posterior wall than other regions (14,37). Previous report showed the posterior LA shares a common embryological origin with the pulmonary veins and possesses unique electrophysiological and structural characteristics that give rise to its arrhythmogenic potential (38). A retrospective study, which included patients with AF underwent CMR in sinus rhythm prior to a PVI procedure, showed a preferential distribution of fibrosis detected by LGE-CMR around the posterior wall surrounding the ostium of the left inferior pulmonary vein (LIPV) (39). It implied permanent trauma caused by the impact of the aortic pulse on the atrial wall may potentially play a role in this fibrosis. Additionally, EAT, especially surrounding left atrium (LA-EAT) has been found to be closely related to LVAs in AF patients, potentially due to myocardial infiltration by fat tissue. Hypertensive patients exhibit significant differences in the volume and attenuation values of EAT around LA compared to non-hypertensive patients. Overall, these studies implied that EAT and other close extracardiac anatomical structures such as AAo, descending aorta may account for increased percentage of LVAs in left atrium (7,40,41). But the difference of distribution of LVAs in AF patients with hypertension remained unclear. We reported a larger percentage of LVAs in AW in hypertensive AF patients. Our findings also demonstrated hypertension may lead to structural changes and dilatation of the ascending aortic, which appear to have a direct relationship with the formation of LVAs in the left atrium.
There are several limitations in our study. Limited by the completeness of data and information, this is a study with a small sample size. In addition, it was an observational study, and we are unable to obtain dynamic changes in HAA associated with hypertension. Therefore, the associations that we observed do not prove the presence of cause and effect relationships. Future large-scale, prospective studies are essential to validate the association between them. Due to limitation in sample size, our study was unable to investigate the association between the severity of hypertension and CoA and LVAs, and future researches could further explore their relationship. What’s more, we employed CoA to represent the degree of LA deformation, thereby indirectly reflecting this compressive effect. However, it lacks experimental evidence, and future experiments should further substantiate this relationship. Future research can explore the difference of AAo-LA relationship whether affects the shear stress, EAT and so on for AF occurrence and recurrence. The specific molecular mechanisms between aortic dilatation caused by hypertension and changes in the electrophysiological properties of the left atrium in patients with AF are worth exploring, and it is necessary to assess the effects of different antihypertensive strategies in preventing or reversing these changes.
Conclusions
In this study, an increase of LVAs in left atrial AW correlated with HAA was observed in hypertensive AF patients. Our study unveils a potential new mechanism by which hypertension influences the onset and recurrence of AF, and offers novel insights into therapeutic strategies for patients with AF who also have hypertension.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2025-80/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2025-80/coif). P.B. reports that this work was supported by the National Natural Science Foundation of China (No. 82270257), and Natural Science Foundation of Shandong Province (No. ZR2021MH011). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (No. KYLL-202411-024), and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2024;45:3314-414. [Crossref] [PubMed]
- Chung MK, Refaat M, Shen WK, Kutyifa V, Cha YM, Di Biase L, Baranchuk A, Lampert R, Natale A, Fisher J, Lakkireddy DR. Atrial Fibrillation: JACC Council Perspectives. J Am Coll Cardiol 2020;75:1689-713. [Crossref] [PubMed]
- Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017;19:891-911. [Crossref] [PubMed]
- Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Brooks AG, Worthington M, Rajendram A, Kelly DR, Zhang Y, Kuklik P, Nelson AJ, Wong CX, Worthley SG, Rao M, Faull RJ, Edwards J, Saint DA, Sanders P. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm 2010;7:1282-90. [Crossref] [PubMed]
- Jansen HJ, McRae MD, Mackasey M, Rose RA. Regional and temporal progression of atrial remodeling in angiotensin II mediated atrial fibrillation. Front Physiol 2022;13:1021807. [Crossref] [PubMed]
- DePaolo J, Levin MG, Tcheandjieu C, Priest JR, Gill D, Burgess S, Damrauer SM, Chirinos JA. Relationship Between Ascending Thoracic Aortic Diameter and Blood Pressure: A Mendelian Randomization Study. Arterioscler Thromb Vasc Biol 2023;43:359-66. [Crossref] [PubMed]
- Guan B, Liu L, Li X, Huang X, Yang W, Sun S, Ma Y, Yu Y, Luo J, Cao J. Association between epicardial adipose tissue and blood pressure: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2021;31:2547-56. [Crossref] [PubMed]
- Batra K, Saboo SS, Kandathil A, Canan A, Hedgire SS, Chamarthy MR, Kalva SP, Abbara S. Extrinsic compression of coronary and pulmonary vasculature. Cardiovasc Diagn Ther 2021;11:1125-39. [Crossref] [PubMed]
- McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J 2024;45:3912-4018. [Crossref] [PubMed]
- Nieman K, García-García HM, Hideo-Kajita A, Collet C, Dey D, Pugliese F, Weissman G, Tijssen JGP, Leipsic J, Opolski MP, Ferencik M, Lu MT, Williams MC, Bruining N, Blanco PJ, Maurovich-Horvat P, Achenbach S. Standards for quantitative assessments by coronary computed tomography angiography (CCTA): An expert consensus document of the society of cardiovascular computed tomography (SCCT). J Cardiovasc Comput Tomogr 2024;18:429-43. [Crossref] [PubMed]
- Hayashida S, Nagashima K, Kurokawa S, Arai M, Watanabe R, Wakamatsu Y, Otsuka N, Yagyu S, Iso K, Okumura Y. Formation of low-voltage zones on the anterior left atrial wall due to mechanical compression by the ascending aorta. J Cardiovasc Electrophysiol 2021;32:2275-84. [Crossref] [PubMed]
- Czerny M, Grabenwöger M, Berger T, Aboyans V, Della Corte A, et al. EACTS/STS Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ. Ann Thorac Surg 2024;118:5-115. [Crossref] [PubMed]
- Schmidt B, Bordignon S, Metzner A, Sommer P, Steven D, Dahme T, Busch M, Tilz RR, Schaack D, Rillig A, Sohns C, Sultan A, Weinmann-Emhardt K, Hummel A, Vogler J, Fink T, Lueker J, Pott A, Heeger C, Chun KRJ. Ablation Strategies for Repeat Procedures in Atrial Fibrillation Recurrences Despite Durable Pulmonary Vein Isolation: The Prospective Randomized ASTRO AF Multicenter Trial. Circulation 2024;150:2007-18. [Crossref] [PubMed]
- Huo Y, Gaspar T, Pohl M, Sitzy J, Richter U, Neudeck S, Mayer J, Kronborg MB, Piorkowski C. Prevalence and predictors of low voltage zones in the left atrium in patients with atrial fibrillation. Europace 2018;20:956-62. [Crossref] [PubMed]
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. [Crossref] [PubMed]
- Kistler PM, Sanders P, Dodic M, Spence SJ, Samuel CS, Zhao C, Charles JA, Edwards GA, Kalman JM. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J 2006;27:3045-56. [Crossref] [PubMed]
- Vio R, Giordani AS, Stefil M, Madine J, Fairbairn T, Themistoclakis S, Salvi P, Caforio ALP, Shantsila A, Shantsila E, Akhtar R, Field M, Lip GYH, Proietti R. Arterial stiffness and atrial fibrillation: shared mechanisms, clinical implications and therapeutic options. J Hypertens 2022;40:1639-46. [Crossref] [PubMed]
- Wang T, Xia YL, Zhang SL, Gao LJ, Xie ZZ, Yang YZ, Zhao J. The impact of hypertension on the electromechanical properties and outcome of catheter ablation in atrial fibrillation patients. J Thorac Dis 2014;6:913-20. [PubMed]
- Kim MH, Kim TH, Hwang I, Park JW, Yu HT, Uhm JS, Joung B, Lee MH, Hwang C, Pak HN. Clinical Characteristics and Rhythm Outcomes in Patients With Atrial Myopathy After Successful Catheter Ablation of Atrial Fibrillation. J Am Heart Assoc 2024;13:e030818. [Crossref] [PubMed]
- Toh N, Kanzaki H, Nakatani S, Ohara T, Kim J, Kusano KF, Hashimura K, Ohe T, Ito H, Kitakaze M. Left atrial volume combined with atrial pump function identifies hypertensive patients with a history of paroxysmal atrial fibrillation. Hypertension 2010;55:1150-6. [Crossref] [PubMed]
- Petre I, Onciul S, Iancovici S, Zamfir D, Stoian M, Scărlătescu A, Diaconeasa A, Acatrinei C, Dorobanțu M. Left Atrial Strain for Predicting Atrial Fibrillation Onset in Hypertensive Patients. High Blood Press Cardiovasc Prev 2019;26:331-7. [Crossref] [PubMed]
- Kloosterman M, Oldgren J, Conen D, Wong JA, Connolly SJ, Avezum A, Yusuf S, Ezekowitz MD, Wallentin L, Ntep-Gweth M, Joseph P, Barrett TW, Tanosmsup S, McIntyre WF, Lee SF, Parkash R, Amit G, Grinvalds A, Van Gelder IC, Healey JS. Characteristics and outcomes of atrial fibrillation in patients without traditional risk factors: an RE-LY AF registry analysis. Europace 2020;22:870-7. [Crossref] [PubMed]
- Kamioka M, Hijioka N, Matsumoto Y, Nodera M, Kaneshiro T, Suzuki H, Takeishi Y. Uncontrolled blood pressure affects atrial remodeling and adverse clinical outcome in paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2018;41:402-10. [Crossref] [PubMed]
- Hsu CC, Chien WC, Wang JC, Chung CH, Liao WI, Lin WS, Lin CS, Tsai SH. Association between Atrial Fibrillation and Aortic Aneurysms: A Population-Based Cohort Study. J Vasc Res 2018;55:299-307. [Crossref] [PubMed]
- Gaudry M, Barral PA, Blanchard A, Palazzolo S, Bolomey S, Omnes V, De Masi M, Carcopino-Tusoli M, Meyrignac O, Rousseau H, Jacquier A, Hassen-Khodja R, Bura-Rivière A, Bartoli JM, Gentile S, Piquet P, Bal L. Prevalence of Thoracic Aortic Aneurysms in Patients with Degenerative Abdominal Aortic Aneurysms: Results from the Prospective ACTA Study. Eur J Vasc Endovasc Surg 2021;61:930-7. [Crossref] [PubMed]
- Nattel S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol 2017;3:425-35. [Crossref] [PubMed]
- Xing M, Chen W, Ji Y, Song W. SLC44A2-mediated phenotypic switch of vascular smooth muscle cells contributes to aortic aneurysm. J Clin Invest 2024;134:e183527. [Crossref] [PubMed]
- Hugas M, Schoenhoff F, Schmidli J, Weiss S. Thoracoabdominal aneurysm causing functional mitral valve stenosis after total arch replacement. Eur J Cardiothorac Surg 2022;62:ezac418. [Crossref] [PubMed]
- Kwon DH, Smedira NG, Popovic ZB, Lytle BW, Setser RM, Thamilarasan M, Schoenhagen P, Flamm SD, Lever HM, Desai MY. Steep left ventricle to aortic root angle and hypertrophic obstructive cardiomyopathy: study of a novel association using three-dimensional multimodality imaging. Heart 2009;95:1784-91. [Crossref] [PubMed]
- Peng Y, Shu X, Lin Y, Huang W, Xu S, Zheng J, Nie R. Anatomical characteristics of aortic valve diseases: Implications for transcatheter aortic valve replacement. Eur J Radiol Open 2023;11:100532. [Crossref] [PubMed]
- Kauhanen SP, Liimatainen T, Kariniemi E, Korhonen M, Parkkonen J, Vienonen J, Vanninen R, Hedman M. A smaller heart-aorta-angle associates with ascending aortic dilatation and increases wall shear stress. Eur Radiol 2020;30:5149-57. [Crossref] [PubMed]
- Sohns C, Lemes C, Metzner A, Fink T, Chmelevsky M, Maurer T, Budanova M, Solntsev V, Schulze WHW, Staab W, Mathew S, Heeger C, Reißmann B, Kholmovski E, Kivelitz D, Ouyang F, Kuck KH. First-in-Man Analysis of the Relationship Between Electrical Rotors From Noninvasive Panoramic Mapping and Atrial Fibrosis From Magnetic Resonance Imaging in Patients With Persistent Atrial Fibrillation. Circ Arrhythm Electrophysiol 2017;10:e004419. [Crossref] [PubMed]
- Bijvoet GP, Hermans BJM, Linz D, Luermans JGLM, Maesen B, Nijveldt R, Mihl C, Vernooy K, Wildberger JE, Holtackers RJ, Schotten U, Chaldoupi SM. Optimal Threshold and Interpatient Variability in Left Atrial Ablation Scar Assessment by Dark-Blood LGE CMR. JACC Clin Electrophysiol 2024;10:2186-97. [Crossref] [PubMed]
- Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol 2005;45:285-92. [Crossref] [PubMed]
- Starek Z, Di Cori A, Betts TR, Clerici G, Gras D, Lyan E, Della Bella P, Li J, Hack B, Zitella Verbick L, Sommer P. Baseline left atrial low-voltage area predicts recurrence after pulmonary vein isolation: WAVE-MAP AF results. Europace 2023;25:euad194. [Crossref] [PubMed]
- Kosiuk J, Dinov B, Kornej J, Acou WJ, Schönbauer R, Fiedler L, Buchta P, Myrda K, Gąsior M, Poloński L, Kircher S, Arya A, Sommer P, Bollmann A, Hindricks G, Rolf S. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm 2015;12:2207-12. [Crossref] [PubMed]
- Wu Y, Qin X, Gao P, Liu Y, Fang Q, Deng H, Cheng K, Cheng Z, Yang D, Chen T. Relationship between the distribution of left atrial low-voltage zones and post-ablation atrial arrhythmia recurrence in patients with atrial fibrillation. Hellenic J Cardiol 2022;66:19-25. [Crossref] [PubMed]
- Suenari K, Chen YC, Kao YH, Cheng CC, Lin YK, Chen YJ, Chen SA. Discrepant electrophysiological characteristics and calcium homeostasis of left atrial anterior and posterior myocytes. Basic Res Cardiol 2011;106:65-74. [Crossref] [PubMed]
- Hopman LHGA, Bhagirath P, Mulder MJ, Eggink IN, van Rossum AC, Allaart CP, Götte MJW. Extent of Left Atrial Fibrosis Correlates with Descending Aorta Proximity at 3D Late Gadolinium Enhancement Cardiac MRI in Patients with Atrial Fibrillation. Radiol Cardiothorac Imaging 2022;4:e210192. [Crossref] [PubMed]
- Natsui H, Watanabe M, Yokota T, Tsuneta S, Fumoto Y, Handa H, et al. Influence of epicardial adipose tissue inflammation and adipocyte size on postoperative atrial fibrillation in patients after cardiovascular surgery. Physiol Rep 2024;12:e15957. [Crossref] [PubMed]
- Sawyer MKL, Gould PA, Ng ACT, Wang WYS. What is the Relationship Between Epicardial Adipose Tissue, Left Atrial Low Voltage Zones and Atrial Fibrillation? Heart Lung Circ 2022;31:1429-31. [Crossref] [PubMed]


