
Reversibility of diffuse magnetic resonance imaging following endovascular therapy in acute ischemic stroke: a systematic review and meta-analysis
Introduction
In the acute phase of ischemic stroke, defined as the time period within minutes of stroke occurrence, diffusion-weighted imaging (DWI) shows strong signals that can identify tissues with limited water diffusion, thereby reflecting the development of cytotoxic edema (1). Magnetic resonance imaging (MRI)-DWI sequences, as the most sensitive sequence for acute ischemic stroke (AIS), highlights evidence of core cerebral infarction (2,3). In clinical practice, a strong DWI signal indicates an infarcted core.
DWI is commonly thought to reflect irreversible tissue damage (4); however, in actual clinical practice, the high-signal area shown on DWI following AIS remains unchanged. One study showed that lesions on DWI may contain salvageable brain tissue comprising a mixture of tissues that ultimately become core infarctions. However, this does not completely represent the infarct core (4). DWI lesions can be partially reversed when reperfusion occurs within a certain time window (5). In addition, many clinical studies have shown that the reversal of high DWI signals in AIS is related to vascular recanalization and reperfusion, which can be seen in patients treated with intravenous thrombolysis (IVT) (6,7) or endovascular therapy (EVT) (8-12), indicating why part of the high signal shown by DWI may represent the ischemic penumbra, which is saved following vascular reperfusion. These conditions trigger part of the DWI high-intensity signal to disappear. In other words, EVT can reduce DWI area expansion (8,13), causing DWI reversal (DWI-R). Moreover, in recent years, with the extension of the EVT time window (up to 16–24 h in some patients), the reversal rate has become higher than that of IVT alone (14,15).
Despite the above research, owing to the relatively small number of patients included in published studies, the estimated prevalences of DWI-R vary widely across the literature, the factors associated with DWI-R have not been fully evaluated, and the relationship between DWI-R and clinical outcomes remains unclear. Therefore, to address this knowledge gap, in the present systematic review and meta-analysis, we investigated the relationship between early EVT and reversibility of DWI in AIS. This study was not prospectively registered and we assessed the studies using the QUADAS-2 quality assessment tool. We present this article in accordance with the PRISMA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2885/rc) (16).
Methods
Search strategy
We systematically analyzed clinical trials evaluating DWI-R after EVT in patients with AIS. The inclusion criteria were conducted according to the Population, Intervention, Comparison, Outcome (PICO) tool (17). Population: people with a diagnosis of AIS. Intervention: clear description of the EVT modality. Comparators: time to initial DWI from stroke onset, time to follow up DWI to evaluate change in stroke size, and time to follow up fluid-attenuated inversion recovery (FLAIR)/T2 performed up to 90 days for assessment of final infarct volume; image acquisition and stroke volumes. Outcomes: volumes of DWI-R and numbers of DWI-R (%) and their association with clinical outcomes.
Search “PubMed, Embase, Web of Science, Medline, Cochrane Library” databases. The literature on the use of EVT for AIS was selected, and the references included in the literature were expanded. The target keywords that we used were various combinations of “acute cerebral infarction”, “acute ischemic stroke”, “ischemic stroke”, “acute ischemic stroke due to large vessel occlusion”, “endovascular”, “endovascular reperfusion”, “endovascular treatment”, “endovascular treatment”, “endovascular therapy”, “thrombectomy”, “emergency treatment”, “reperfusion”, “reperfusion treatment”, “diffusion weighted imaging”, “DWI”, “reversal”, “reversibility”, “DWI reversal”. The retrieval period was from the establishment of each database until June 1, 2024. The titles and abstracts generated through the initial database search were first analyzed, and the literatures that were clearly not compatible were excluded. Then, the full text of the literatures after preliminary screening were obtained to further evaluate whether they met the inclusion criteria. The detailed search strategy is presented in Appendix 1.
Study selection (Table 1)
Table 1
| Studies were included if they met all the following criteria |
| (I) Enrolled patients with age ≥18 years old |
| (II) Symptoms/signs of focal neurological impairment could be clearly observed |
| (III) Baseline core infarct volume ≥5 mL |
| (IV) Patients with AIS who underwent EVT |
| (V) Baseline MRI was performed within 24 hours, and follow-up MRI was performed within 1 week |
| (VI) The included studies detailed the changes in infarct volume before and after EVT |
| (VII) Original clinical studies and access to the full text |
| (VIII) Successful recanalization with EVT |
| (IX) Neuroimaging results showing that EVT was caused by the occlusion of large blood vessels |
| Studies were excluded if they met any of the following criteria |
| (I) Duplicate studies |
| (II) Research content is inconsistent |
| (III) Head CT was used to evaluate the final infarct volume |
| (IV) Animal studies |
| (V) Review, meta-analysis, or systematic review design |
| (VI) Conference abstracts |
| (VII) Comments |
| (VIII) Case reports |
| (IX) Letters |
AIS, acute ischemic stroke; CT, computed tomography; EVT, endovascular therapy; MRI, magnetic resonance imaging.
Data extraction and analysis
Two researchers (E.L.L. and S.M.L., both are master’s degree holders) independently conducted literature searches according to pre-established criteria, with screening performed according to the inclusion and exclusion criteria. The full text of all studies which met the inclusion criteria was read, and the following relevant data were extracted: author, publication year, journal name, study type (prospective or retrospective), study country, age of study subjects, whether the selection of cases was continuous, study sample size, and level of evidence. For the latter, the evidence grading and recommendation strength standards formulated by the Center for Evidence-Based Medicine of the University of Oxford were adopted, and the accepted evidence was divided into five levels (I to V) according to the reliability of the accepted evidence (18). The selection of the above studies and extraction of relevant materials and data were independently completed by two researchers. Any disagreements were resolved through negotiations with a third researcher (A.W., a master’s degree holder).
This was a single rate meta-analysis with data compiled from the included literature, with the primary objective of determining the proportion of patients with DWI-R. DWI-R was defined as a decrease in DWI lesion volume within 7 days of follow-up or FLAIR/T2 lesion volume within 90 days of follow-up compared with the baseline DWI lesion volume within 24 h after stroke (19,20). Further, specific variables related to the intervention were also collected, including the interval between symptom onset and EVT, description of occlusive vessels, imaging protocols and characteristics, National Institutes of Health Stroke Scale (NIHSS) score, intervention mode, and number of patients with DWI-R. Normally distributed measurement data are represented as the mean ± standard deviation, while non-normally distributed data are shown as the median (interquartile). Counting data is expressed as the frequency (percentage).
Quality assessment and statistical analysis
The quality of the studies was independently assessed by two reviewers using Review Manager 5.3 software. Statistical analyses were performed using STATA 15.0. Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criterion was used to evaluate the risk of bias and applicability concerns of all the included studies (21), and a literature quality evaluation chart was drawn. Discrepancies were resolved through a discussion with the third investigator.
Results
Literature search results and characteristics of the included studies
A total of 515 relevant studies were initially retrieved, of which three were obtained by manual reference examination. The titles/abstracts of the articles were preliminarily reviewed, and 430 were excluded because they were not relevant to the research topic. A total of 85 articles were evaluated as qualified. After application of the exclusion criteria, five studies (8-12) published between 2014 and 2023 were included in the analysis. The literature retrieval process is illustrated in Figure 1. Four (8,10-12) of the included studies had level II evidence (80%), and one (9) had level III evidence (20%). The inclusion of all cases was continuous, including four retrospective (8,9,11,12) studies and one prospective (10) study. The total number of patients in each study ranged from 60 to 433, with 1,226 total patients from six countries enrolled. Occluded blood vessels included the internal carotid artery and M1/M2/M3 middle cerebral arteries (MCAs). Four studies (9-12) used the IVT reperfusion therapy. The median NIHSS score at baseline was 15–20. The average time from stroke onset to EVT was 190–354 min. The proportion of men ranged from 45% to 58.6%, with a mean age of 62–76 years and median age of 68 years. The basic characteristics and relevant data of the included studies are presented in Tables 2,3.
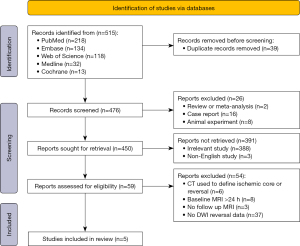
Table 2
| Author | Year | Study design | Patient selection | Sample size, n | Male | Age (years) | Level of evidence | Journal |
|---|---|---|---|---|---|---|---|---|
| Inoue (8) | 2014 | Retrospective | Random | 60 | 27 (45%) | 62±13† | II | Stroke |
| Yoo (9) | 2019 | Retrospective | Consecutive | 404 | 225 (55.7%) | 67.6±12.2† | III | Stroke |
| Panni (10) | 2022 | Prospective | Consecutive | 211 | 124 (58.6%) | 65±15† | II | Clin Neuroradiol |
| Umemura (12) | 2022 | Retrospective | Consecutive | 118 | 62 (53%) | 76±11† | II | AJNR Am J Neuroradiol |
| Scopelliti (11) | 2023 | Retrospective | Consecutive | 433 | 205 (47%) | 68 (53.5–80)‡ | II | Stroke |
†, mean ± SD; ‡, median or median (IQR). IQR, interquartile range; SD, standard deviation.
Table 3
| Study | Country | Intervention | Time from stroke onset to EVT/arterial puncture | Number of DWI-R (%) | Baseline NIHSS score | Key points |
|---|---|---|---|---|---|---|
| Inoue et al. 2014 (8) | America | EVT | 354 (240–474) min† | 19 (32%) | 17 (12–21)† | Early DWI-R was often transient in EVT, and was not associated with good LTO |
| Yoo et al. 2019 (9) | Korea | t-PA: 188 (55.1%) + EVT§/t-PA: 35 (55.6%) + EVT¶ | 338±222‡ min | 63 (15.5%) | 16 (12–20)†§/15 (12–19)†¶ | Complete reperfusion and early reperfusion therapy were associated with DWI-R |
| Panni et al. 2022 (10) | France, Italy, and Greece | t-PA + EVT | 225±127‡ min | 39 (18.9%) | 17 (15–20)†§/19 (16–22)†¶ | DWI-R can significantly affect clinical prognosis; the mTICI 2c–3 recirculation is an independent predictor of DWI-R |
| Umemura et al. 2022 (12) |
Japan | t-PA + EVT | 190 (112–270) min† | 42 (35.6%) | 18 (14–23)† | ADC value is easy to obtain, a useful tool for predicting survival lesions, and is independently correlated with DWI reversal |
| Scopelliti et al. 2023 (11) |
France | t-PA: 283 (65.4%) + EVT | 270 (196–321)†#/291 (220–342)†^ | 22% (patients ≥80) and 19% (patients <80) | 20 (16–23)†#/17 (13–20)†^ | DWI-R after MT is affected by arterial recanalization, is associated with good outcomes, and is common in both older and younger stroke patients |
†, median or median (IQR); ‡, mean ± SD; §, non-DWI-reversal group; ¶, DWI-reversal group; #, patients ≥80 years; ^, patients <80 years. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; DWI-R, diffusion-weighted imaging reversal; EVT, endovascular therapy; IQR, interquartile range; LTO, long-term outcome; NIHSS, National Institutes of Health Stroke Scale; MT, mechanical thrombectomy; mTICI, modified Thrombolysis in Cerebral Infarction; SD, standard deviation; t-PA, tissue plasminogen activator.
Study quality and risk of bias
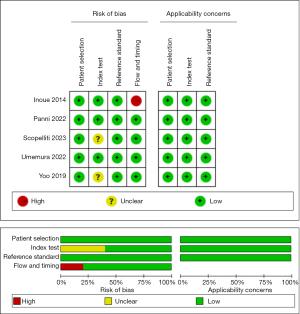
The QUADAS-2 tool was used to assess the quality of all eligible studies in terms of four aspects: patient selection, index test, reference standard, and flow and timing (Figure 2). As the selected studies included consecutive or randomly selected patients, the risk of bias in terms of patient selection was assessed as low. The risk of bias in the index test was unclear in two studies, and the other study was judged to have a high risk of bias in the flow and timing domain. All studies used the baseline DWI imaging within 24 h of stroke as the reference standard for diagnosing ischemic core, which could correctly distinguish the target disease and the risk of bias in the reference standard was assessed as low (detail results given in Table S1).
Imaging protocols and characteristics for included studies
The five studies included in this review mainly adopted different evaluation criteria according to the time of follow-up imaging and volume reduction. Inoue et al. enrolled investigators who underwent MRI before (DWI 1) and within 12 h after (DWI 2) EVT and MRI follow-up on day 5 (8). The authors concluded that a volume >10 mL of a DWI 1 lesion that did not overlap with a DWI 2 lesion was defined as early DWI-R, while lesions with a volume >10 mL that did not overlap with a FLAIR lesion on day 5 was defined as permanent DWI-R. Yoo et al. conducted an MRI follow-up 3–7 days after EVT, defining DWI-R in patients in whom the DWI volume at follow-up was reduced compared with the baseline DWI volume (9). Panni et al. proposed that when the MRI was reviewed 24 h post-EVT, if the high signal involving at least one DWI Alberta Stroke Program Early CT Score (ASPECTS) area was completely degenerated, it could be considered as reversal (10). Scopelliti et al. defined certain regions as bright on baseline DWI images, defining them as DWI-R if the DWI showed the normal presence of parenchymal voxels after 24 h of follow-up (11). Finally, one study showed that DWI-R could be summarized as high intensity at baseline DWI, but no subsequent FLAIR (12) (Table 4).
Table 4
| First author, YOP | Scanner strength | MRI protocol and timing | Vascular territory | DWI volume (mL) | Method of volume assessment | DWI-R definition |
|---|---|---|---|---|---|---|
| Inoue et al., 2014 (8) | 1.5 or 3 T | DWI, PWI, MRA head MR1: baseline MR2: 12 h after EVT MR3: discharge or 5 days—DWI, GRE and FLAIR |
ICA or MCA | MR1 DWI: 26.8 (13.8–56.9)† | ROIs with MIPAV software and calculating DWI high-signal regions and ADC values | Early DWI-R: MR1 DWI lesion not superimposed on MR2 DWI at 12 h >10 mL |
| MR1 DWI: 26.8 (13.8–56.9)† | Early DWI-R: MR1 DWI lesion not superimposed on MR2 DWI at 12 h >10 mL | |||||
| Yoo et al., 2019 (9) | NA | DWI only MR1: baseline MR2: 3 to 7 days after EVT |
Intracranial ICA or MCA-M1 or MCA-M2 | Baseline MR1 DWI: 30.1±36.7‡ (DWI-R group) versus 22.0±30.7‡ (no DWI-R group) | DWI high-signal regions and ADC values were calculated by NordiceICE semiautomated software | DWI-R: decrease in lesion volume on DWI at 3–7 days compared to baseline DWI lesion within 24 hours of stroke |
| Follow-up MR2 DWI: 17.8±24.9‡ versus 68.7±77.5‡ | ||||||
| Panni et al., 2022 (10) | NA | DWI, FLAIR, T2, TOF MRA head MR1: baseline MR2: 24 h after thrombectomy |
ICA terminus occlusion or tandem occlusion or MCA-M1 or MCA-M2 | NA | Change in stroke volume on ADC (with ADC value <615×10−6 mm2/s) at 24 h from thrombectomy | DWI-R: high DWI signals involve complete regression of at least one DWI ASPECTS region |
| Umemura et al., 2022 (12) | 3 T | DWI/ADC, T2, FLAIR, MRA head MR1: baseline MR2: 23–27 h after MT |
ICA, M1, M2, M3 | NA | Manual ROIs and calculating ADC values | DWI-R: hyper intense on baseline DWI but not on follow-up FLAIR |
| Scopelliti et al., 2023 (11) | 1.5 T | DWI/ADC, T2 MR1: baseline MR2: 24 h post MT |
ICA or MCA-M1 or MCA-M2 | Baseline MR1 DWI: 29.1 (13.8–68.0)† (≥80 Y) 31.9 (17.5–59.0)† (<80 Y) |
DWI high-signal regions and ADC values were calculated by Mango software | DWI-R: clearly visible initial hyperintensities that were no longer visible on follow-up DWI at 24 h |
| Follow-up MR2 DWI: 74.5 (25.1–131.1)† (≥80 Y) 61.7 (30.0–122.6)† (<80 Y) |
†, median or median (IQR); ‡, mean ± SD. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; DWI-R, diffusion-weighted imaging reversal; EVT, endovascular therapy; FLAIR, fluid attenuated inversion recovery; GRE, gradient recalled echo; ICA, internal carotid artery; IQR, interquartile range; MCA, middle cerebral artery; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging; MR1, first consecutive MRI performed in the study; MR2, second consecutive MRI performed in the study; MR3, third consecutive MRI performed in the study; MT, mechanical thrombectomy; NA, no available data; PWI, perfusion weighted imaging; ROIs, regions of interest; SD, standard deviation; T2, T2 weighted MRI; Y, years; YOP, year of publication.
Meta-analysis
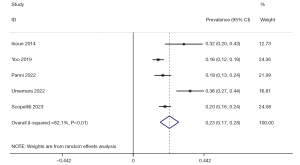
The I2 value and Cochrane Q test results showed significant heterogeneity among the included studies (I2=82.1%, P<0.01). As a result, a random-effects model was used for the meta-analysis. The pooled prevalence of DWI-R after EVT was 0.23 [95% confidence interval (CI): 0.17–0.28] (Figure 3).
The publication bias of the included studies was determined using Egger’s funnel plot, from which P=0.062>0.05 was obtained, indicating that there was no significant publication bias among the included literatures in this study (Figure 4).
Correlation between ASPECT-CT/MRI score and DWI-R
Panni et al. conducted a national prospective multicenter study (10). Patients with consecutive anterior circulation infarction with pre-onset modified Rankin scale (mRS) score values ≤2 and baseline DWI-ASPECTS 0–5 were enrolled. Follow-up MRI was performed 24 h after EVT, and the incidence of DWI-R was 18.5%. DWI-R was identified as an independent predictor of early neurological improvement (ENI) and was associated with improved 90-day clinical functional outcomes.
Apparent diffusion coefficient (ADC) levels and DWI-R
A retrospective study examined the association between ADC values and DWI-R in patients undergoing EVT. Umemura et al. included 118 patients, among whom 42 (36%) developed DWI-R (12). The mean, maximum, and minimum ADC values in the lesion reversal zone of DWI were 575×10−6, 747×10−6, and 441×10−6 mm2/s, respectively, which were significantly higher than those in the non-DWI lesion reversal zones (478×10−6, 641×10−6, and 346×10−6 mm2/s, respectively). Receiver operating characteristic (ROC) curve analysis showed that the optimal ADC cutoff value for distinguishing the core area of cerebral infarction from the area of lesion reversal was 520×10−6 mm2/s, with a prediction accuracy of 82%, sensitivity of 71%, and specificity of 83%. Multivariate analysis revealed that ADC was an independent predictor of DWI-R after reperfusion. Compared with the NIHSS score at 7 days, patients achieving DWI-R had earlier neurological function improvement, and the functional independence rate of patients at 3 months was significantly higher. Compared to patients without DWI-R, both the time from onset-to-imaging and onset-to-revascularization were significantly shorter in patients with DWI-R.
Patient age and DWI-R
The percentage of DWI-R (DWI-R%), was calculated as follows: DWI-R% = (DWI-R volume/baseline DWI volume) × 100. Scopelliti et al.’s study included 433 patients (median age 68 years), and the median DWI-R% after EVT was 22% in patients ≥80 years and 19% in patients <80 years (11). In the multivariate model, DWI-R% was significantly associated with 3-month clinical outcomes in the two groups of patients of different ages (11). For both groups, successful reperfusion had a significant effect on DWI-R, whereas collateral vessel status and WMH volume showed no significant association with DWI-R% (11). In both groups, the DWI-R% was associated with an increased rate of good functional outcomes at 3 months, and the estimated benefit appeared to be independent of age. The study also found that lesion regression was the only factor other than the baseline NIHSS score that was significantly associated with an increased rate of favorable post-stroke outcomes in older patients. However, this study did not find any difference in baseline infarct size between younger and older patients, and the positive association between DWI-R% and 3-month good functional outcomes remained even after adjusting for baseline infarct size.
Predictors of DWI-R and association of DWI-R with clinical outcomes
ENI has been defined as a decrease of ≥4 from baseline NIHSS score at 24 h after EVT (10). In addition, Panni et al. showed that DWI-R was an independent predictor of improvement in ENI and mRS scores (10). Binary logistic regression analysis showed that a modified Thrombolysis in Cerebral Infarction (mTICI) scale score of 2c–3 reproducibility was the only factor that independently predicted DWI-R (10). Sakamoto et al. further found that early reclosing was related to DWI-R, and that the DWI-R group was more likely to achieve complete reperfusion (22). In this study, 24.8% of patients with mTICI 3 showed DWI-R, whereas the DWI-R rate in patients with mTICI 2b was only 12.1%. Moreover, the functional outcomes of patients with DWI-R at 3 months were better than those of the latter group, suggesting that complete recanalization enables the salvage of functionally reversible tissue, which in turn promotes DWI-R formation, ultimately leading to better clinical outcomes. In addition, the study also found that patients with DWI-R had a higher incidence of atrial fibrillation than those without reversal. Sakamoto et al. further found that young age, lower initial NIHSS scores, lower baseline DWI volumes, shorter initial MRI to final reperfusion times, and complete reperfusion were independently associated with good functional outcomes (22). Multivariate analysis further revealed that complete reperfusion and a shorter time interval from imaging to final reperfusion were independent predictors of DWI-R.
Discussion
DWI lesions commonly represent cellular energy depletion and early cytotoxic edema and are generally considered to be markers of irreversible ischemic changes in AIS patients (11). However, many reports have shown that DWI lesions are reversible in the early stages of AIS (23); that is, some high-signal areas on DWI may not represent irreversibly damaged ischemic tissue (24,25). Following the procedure, reperfusion therapy is administered; reperfusion therapy appears to promote DWI-R, challenging the conventional view that DWI-positive areas represent irreversible damage and repair, and promising better outcomes after stroke in patients undergoing EVT (9). Therefore, identifying the irreversible infarct core is critical to assess the benefits and risks of EVT among patients with AIS.
Previous studies have suggested that reversal of cerebral infarction lesions, particularly sustained reversal, is rare (26). Early DWI-R may therefore be caused by a temporary increase in ADC values following brain tissue reperfusion, which may be related to vasogenic edema rather than effective brain tissue rescue (27). Studies have further suggested that DWI lesions, especially early lesions, are not equivalent to ischemic cores (28,29). Both laboratory and clinical studies have shown that even when early reperfusion is achieved, tissue retention in the co-registered area of acute DWI lesions is limited (30). Asdaghi et al. have further reported that DWI-R often occurs in patients with transient ischemic attack (TIA) or minor stroke (4). Albach et al. concluded that DWI high-signal completely reversible lesions in AIS are more common in small embolic cases (31). In addition, it has been suggested that permanent DWI-R commonly involves only a small amount of brain tissue (26,32). Inoue et al. reported that the median permanent reversal volume of cerebral infarction was 3 mL (8). This study also found that for those who underwent intravascular therapy and reperfusion, the volume of infarct which spread to neighboring tissues was much larger than that for those who achieved permanent reversal, indicating the growth of lesions in one area and the reversal of lesions in another area. There may be no net volume change, resulting in a final infarct size equal to the baseline DWI volume (8). We found that the different studies on DWI-R rate differences were very large, ranging from 7% to 85% (24,26,33), possibly because DWI-R can define different baseline and follow-up MRI imaging times.
Owing to changes in ADC values following reperfusion, in addition to the development of vasogenic edema, the ideal time for DWI assessment remains controversial. If follow-up imaging is performed within a few hours of reperfusion, the rapid increase in ADC values can mask the change in DWI values, resulting in the incorrect assumption that the brain tissue has been saved (24). This idea is supported by basic studies in animal models that confirmed the occurrence of new infarcts in these areas through imaging follow-up or pathological examination following reperfusion (30,34). In addition, preclinical studies in animal models have shown that DWI-R areas often show subtle changes on delayed T2 or FLAIR imaging, which may not be infarcts, but may involve selective neuronal necrosis (5).
The DEFUSE trial noted that the time from disease onset to puncture has traditionally been emphasized (6). In recent years, with the deepening of research in the EVT era, an increasing number of studies have indicated the superior importance of reperfusion time (35,36). For example, Yoo et al. highlighted the importance of the time from stroke onset to actual reperfusion (9). Other studies have suggested that image acquisition-to-reperfusion time is closely related to patient prognosis (37,38). It should be noted that the time from imaging to reperfusion varies according to the hospital setting and patient condition (39,40). In addition, Sunde et al. found that 70.8% of 24-h DWI-R persisted at a later time point (2–3 days) and were independently associated with ENI (41).
Currently, there are relatively few studies on DWI-R for patients with a low ASPECTS. In some studies, neither baseline ischemic expansion (DWI-ASPECTS) nor neurological severity (NIHSS) were found to be associated with DWI-R (9,26). Conversely, other studies have suggested that successful recalculation and functional collateral status mediate an increase in edema absorption and a decrease in the need for decompression craniotomy for low CT-ASPECTS (42-44). Further, other studies have pointed out that after EVT, 25% to 30% of patients with low ASPECTS points or large volumes of stroke may achieve good outcomes (defined as 90-day mRS 0–2) (44-46).
ADC, which is commonly used as a measure of the diffusion of water molecules in tissues, is reduced in ischemic tissues owing to cytotoxic edema (47). A reduction in ADC is associated with decreased energy metabolism, which eventually leads to core infarction (48,49). According to Campbell et al., diffusion restriction suggests irreversible ischemic injury (26); Purushotham et al. proposed that the ADC threshold in the infarct core area was ≤620×10−6 mm2/s (50). In regards to the ADC threshold, Shinoda et al. believed that a high relative ADC ratio (that is, the ratio of ADC value in the lesion area to ADC value in the contralateral normal brain area) could lead to DWI-R, and further found that the average relative ADC ratio in the lesion area of reversible DWI was 0.890 (51). The mean relative ADC ratio of the lesion area on the final infarction DWI was 0.640. Another study suggested that the ADC value of the reversible part of DWI was higher than that of the irreversible part (20,52), whereas the ADC value of the irreversible part of the DWI was higher than that of a previously defined diffuse lesion (ADC threshold, <620) (50). The reversal of persistent DWI lesions has previously been demonstrated in animal models using temporary arterial occlusion (53,54). These findings indicate that a mild ADC reduction before metabolic energy depletion could be reversed by early reperfusion therapy (51,55).
Partial DWI-R after EVT is common in both older and younger patients with stroke, is significantly affected by arterial recanalization, and is associated with an increased rate of good outcomes following stroke (11). In fact, previous studies have shown that good outcomes in older patients are largely dependent on salvaged brain tissue, whereas younger patients may develop additional coping mechanisms to achieve good functional independence after stroke (56). Therefore, in future clinical studies, achieving the reversal of DWI lesions should be regarded as an important therapeutic goal and evaluation parameter, especially in elderly patients. Nevertheless, it has been suggested that ADC is not a good biomarker that reliably reflects true tissue diffusion (57). ADC measurements are susceptible to perfusion, tissue structure, cell structure, T2* effect, scanning parameters and many other factors (58). The changes of ADC after cerebral ischemia are time-dependent, showing a high dependence on T2 (57). The study of Wáng et al. suggested that the tri-phasic relationship between T2 time and ADC provided complementary information for the evolution of ischemia and jointly optimized diagnosis and prognosis assessment (59). Therefore, we believe that continuous follow-up of ADC and T2 changes will help distinguish between reversible injury and irreversible infarction.
It has previously been suggested that early DWI-R is not an independent predictor of clinical outcomes, and is not associated with early reperfusion (8). In addition, the reversal of diffuse lesions does not necessarily imply complete tissue salvage (5). However, other studies have shown that a repairable ischemic core and penumbra could cause diffuse changes; therefore, the reversal of DWI is of great clinical significance (22). DWI-R has been confirmed in several preclinical studies using transient MCA occlusion models, which assume complete reperfusion. Indeed, several studies have indicated that the main goal of EVT in clinical practice is to achieve mTICI grade 2b recanalization, rather than complete reperfusion (5,47). However, other studies have shown that reperfusion to mTICI 2b is inadequate; for example, blood flow to mTICI 3 following thrombolysis in cerebral infarction shows better clinical outcomes than mTICI 2b (60-64). Gilgen et al. further studied thrombectomy for large-volume (>70 mL) DWI lesions, and one out of three patients achieved complete reperfusion and had a good prognosis (65). Only one in 12 patients with poor or failed reperfusion had good prognosis. Thus, clinicians should aim to achieve complete reperfusion (mTICI 3) rather than successful reperfusion (mTICI 2b–3) when treating AIS, in addition to minimizing the time from imaging to reperfusion (9). Yoo et al. further noted that early treatment and complete reperfusion are associated with DWI-R and are important imaging factors for good functional outcomes (9,24). Throughout the above studies, the prognosis of DWI-R was quite different, possibly due to differences in the characteristics of patients, different lesion volumes of DWI, different treatment methods, status of collateral circulation, and early opening time between the studies (20). In addition, lesion location may affect prognosis (5).
Limitations of the included studies
This meta-analysis review evaluated the limitations of the included studies based on the following aspects:
- Heterogeneity in study design: some studies were prospective; however, some were retrospective, and there was a selection bias in the selection of cases.
- Different reference criteria: different thresholds for the reversibility of the core infarct area were set, meaning that different definitions of DWI-R were used, which could have limited the interpretation of the reported results.
- Limitations of the study methods: some studies did not use randomization, making it difficult to accurately assess the incidence of DWI-R between those with and without EVT, which may have affected the reliability of the study results.
- Sample selection and representativeness: the median baseline DWI lesion volume in some studies was relatively small, making it difficult to predict the reversal rate of patients with large lesions on baseline DWI. Patients with severe stroke or early death were excluded because of the difficulty in obtaining baseline or follow-up MRI data, which did not fully reflect the prognosis of these patients and invisibly overestimated the proportion of DWI-Rs.
- Data collection and analysis: there were significant differences in the methods used to calculate and analyze the infarct volume of DWI and FLAIR, including simple artificial regions of interest and volume measurement techniques, and complex voxel-based co-registration and segmentation methods, leading to deviations in the consistency of measurement results, affecting the reversal rate and volume of DWI. Further, some studies only recorded the total volume of the DWI-positive region and did not measure the different components or lesions in the high-signal regions of DWI and their effect on reversal.
- Other variables that may affect DWI-R include age at disease onset, distribution of vascular occlusion sites, status of collateral vessels, time from onset to imaging, time from imaging to vascular reperfusion, and follow-up time.
Overall, various MRI methods have been reported for selecting ischemia/salvable areas for reperfusion therapy (66-68). Selecting patients according to perfusion-lesion-ischemic core mismatch has become a research hotspot (14,69,70). The key to this tissue window-based decision-making method is to evaluate the size of the irreversible core infarction and the salvageable ischemic penumbra. In clinical practice, in the same patient, reversible changes occur in part of the core infarction area between two imaging examinations before and after EVT, while some ischemic penumbra may progress into the infarct core, making it difficult to truly reflect the reversible changes in the core infarction area. If the volume of the reduced core infarction re-examined after surgery is used to judge the reversibility of DWI, it would have a more direct clinical reference value. Further, collateral circulation is an important factor in the reversibility of baseline core infarction. In the five studies included in this review, patients with vascular occlusion in different parts of the internal carotid artery and M1/M2/M3 MCA were enrolled, and it was difficult to achieve a uniform assessment of the collateral circulation status. In the real world, clinicians focus on the extent to which patients can reduce their final total infarct volume based on baseline imaging assessment through reperfusion techniques such as intravascular interventions and do not consider whether this reduction is simply the result of reversible changes or is partially offset by focal progression in the ischemic penumbra.
Conclusions
Overall, this study confirmed that a high-intensity DWI signal does not fully represent the infarct core. In clinical practice, modern reperfusion techniques such as EVT often achieve a high DWI-R, which is closely related to ENI and 90-day clinical functional outcomes. Therefore, clinicians should be cautious when making clinical decisions based on DWI lesion volumes. Large-scale prospective clinical studies are needed to further clarify and comprehensively evaluate the relationship between early EVT and DWI-R in patients with AIS.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2885/rc
Funding: This work was sponsored by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2885/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab 2016;36:513-38. [Crossref] [PubMed]
- Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, Kidwell CS, Mohr JP, Sloan M, Sorensen AG, Warach STherapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke [RETIRED]: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;75:177-85. Erratum in: Neurology 2010;75:938.
- Provost C, Soudant M, Legrand L, Ben Hassen W, Xie Y, Soize S, Bourcier R, Benzakoun J, Edjlali M, Boulouis G, Raoult H, Guillemin F, Naggara O, Bracard S, Oppenheim C. Magnetic Resonance Imaging or Computed Tomography Before Treatment in Acute Ischemic Stroke. Stroke 2019;50:659-64. [Crossref] [PubMed]
- Asdaghi N, Campbell BC, Butcher KS, Coulter JI, Modi J, Qazi A, Goyal M, Demchuk AM, Coutts SB. DWI reversal is associated with small infarct volume in patients with TIA and minor stroke. AJNR Am J Neuroradiol 2014;35:660-6. [Crossref] [PubMed]
- Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, Fisher M, Hsu CY, Lin W. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke 2000;31:946-54. [Crossref] [PubMed]
- Olivot JM, Mlynash M, Thijs VN, Purushotham A, Kemp S, Lansberg MG, Wechsler L, Bammer R, Marks MP, Albers GW. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke 2009;40:1692-7. [Crossref] [PubMed]
- Soize S, Tisserand M, Charron S, Turc G, Ben Hassen W, Labeyrie MA, Legrand L, Mas JL, Pierot L, Meder JF, Baron JC, Oppenheim C. How sustained is 24-hour diffusion-weighted imaging lesion reversal? Serial magnetic resonance imaging in a patient cohort thrombolyzed within 4.5 hours of stroke onset. Stroke 2015;46:704-10. [Crossref] [PubMed]
- Inoue M, Mlynash M, Christensen S, Wheeler HM, Straka M, Tipirneni A, Kemp SM, Zaharchuk G, Olivot JM, Bammer R, Lansberg MG, Albers GW. DEFUSE 2 Investigators. Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke 2014;45:1024-8. [Crossref] [PubMed]
- Yoo J, Choi JW, Lee SJ, Hong JM, Hong JH, Kim CH, Kim YW, Kang DH, Kim YS, Hwang YH, Ovbiagele B, Demchuk AM, Lee JS, Sohn SI. Ischemic Diffusion Lesion Reversal After Endovascular Treatment. Stroke 2019;50:1504-9. [Crossref] [PubMed]
- Panni P, Lapergue B, Maïer B, Finitsis S, Clarençon F, Richard S, et al. Clinical Impact and Predictors of Diffusion Weighted Imaging (DWI) Reversal in Stroke Patients with Diffusion Weighted Imaging Alberta Stroke Program Early CT Score 0-5 Treated by Thrombectomy: Diffusion Weighted Imaging Reversal in Large Volume Stroke. Clin Neuroradiol 2022;32:939-50. [Crossref] [PubMed]
- Scopelliti G, Benzakoun J, Ben Hassen W, Bretzner M, Bricout N, Puy L, Turc G, Boulouis G, Oppenheim C, Naggara O, Cordonnier C, Henon H, Pasi M. Diffusion-Weighted Imaging Lesion Reversal in Older Patients With Stroke Treated With Mechanical Thrombectomy. Stroke 2023;54:1823-9. [Crossref] [PubMed]
- Umemura T, Hatano T, Ogura T, Miyata T, Agawa Y, Nakajima H, Tomoyose R, Sakamoto H, Tsujimoto Y, Nakazawa Y, Wakabayashi T, Hashimoto T, Fujiki R, Shiraishi W, Nagata I. ADC Level is Related to DWI Reversal in Patients Undergoing Mechanical Thrombectomy: A Retrospective Cohort Study. AJNR Am J Neuroradiol 2022;43:893-8. [Crossref] [PubMed]
- Xie Y, Oppenheim C, Guillemin F, Gautheron V, Gory B, Raoult H, Soize S, Felblinger J, Hossu G, Bracard STHRACE investigators. Pretreatment lesional volume impacts clinical outcome and thrombectomy efficacy. Ann Neurol 2018;83:178-85. [Crossref] [PubMed]
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018;378:708-18. [Crossref] [PubMed]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2018;378:11-21. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.
- Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 2014;14:579. [Crossref] [PubMed]
- Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am 2003;85:1-3.
- Lakomkin N, Pan J, Stein L, Malkani B, Dhamoon M, Mocco J. Diffusion MRI Reversibility in Ischemic Stroke Following Thrombolysis: A Meta-Analysis. J Neuroimaging 2020;30:471-6. [Crossref] [PubMed]
- Nagaraja N, Forder JR, Warach S, Merino JG. Reversible diffusion-weighted imaging lesions in acute ischemic stroke: A systematic review. Neurology 2020;94:571-87. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Sakamoto Y, Kimura K, Shibazaki K, Inoue T, Uemura J, Aoki J, Sakai K, Iguchi Y. Early ischaemic diffusion lesion reduction in patients treated with intravenous tissue plasminogen activator: infrequent, but significantly associated with recanalization. Int J Stroke 2013;8:321-6. [Crossref] [PubMed]
- Goyal M, Ospel JM, Menon B, Almekhlafi M, Jayaraman M, Fiehler J, et al. Challenging the Ischemic Core Concept in Acute Ischemic Stroke Imaging. Stroke 2020;51:3147-55. [Crossref] [PubMed]
- Labeyrie MA, Turc G, Hess A, Hervo P, Mas JL, Meder JF, Baron JC, Touzé E, Oppenheim C. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke 2012;43:2986-91. [Crossref] [PubMed]
- Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Schlaug G, Bammer R, Marks MP, Albers GW. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE). Stroke 2008;39:2257-63. [Crossref] [PubMed]
- Campbell BC, Purushotham A, Christensen S, Desmond PM, Nagakane Y, Parsons MW, Lansberg MG, Mlynash M, Straka M, De Silva DA, Olivot JM, Bammer R, Albers GW, Donnan GA, Davis SM. EPITHET–DEFUSE Investigators. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab 2012;32:50-6. [Crossref] [PubMed]
- Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME. Evaluation of early reperfusion and i.v. tPA therapy using diffusion- and perfusion-weighted MRI. Neurology 1999;52:1792-8. [Crossref] [PubMed]
- Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol 2009;30:1206-12. [Crossref] [PubMed]
- Guadagno JV, Warburton EA, Aigbirhio FI, Smielewski P, Fryer TD, Harding S, Price CJ, Gillard JH, Carpenter TA, Baron JC. Does the acute diffusion-weighted imaging lesion represent penumbra as well as core? A combined quantitative PET/MRI voxel-based study. J Cereb Blood Flow Metab 2004;24:1249-54. [Crossref] [PubMed]
- Henninger N, Sicard KM, Fisher M. Spectacular shrinking deficit: insights from multimodal magnetic resonance imaging after embolic middle cerebral artery occlusion in Sprague-Dawley rats. J Cereb Blood Flow Metab 2007;27:1756-63. [Crossref] [PubMed]
- Albach FN, Brunecker P, Usnich T, Villringer K, Ebinger M, Fiebach JB, Nolte CH. Complete early reversal of diffusion-weighted imaging hyperintensities after ischemic stroke is mainly limited to small embolic lesions. Stroke 2013;44:1043-8. [Crossref] [PubMed]
- Chemmanam T, Campbell BC, Christensen S, Nagakane Y, Desmond PM, Bladin CF, Parsons MW, Levi CR, Barber PA, Donnan GA, Davis SM. EPITHET Investigators. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology 2010;75:1040-7. [Crossref] [PubMed]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol 2000;47:462-9.
- Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke 2001;32:2362-9. [Crossref] [PubMed]
- Hwang YH, Kang DH, Kim YW, Kim YS, Park SP, Liebeskind DS. Impact of time-to-reperfusion on outcome in patients with poor collaterals. AJNR Am J Neuroradiol 2015;36:495-500. [Crossref] [PubMed]
- Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA 2016;316:1279-88. [Crossref] [PubMed]
- d'Esterre CD, Boesen ME, Ahn SH, Pordeli P, Najm M, Minhas P, Davari P, Fainardi E, Rubiera M, Khaw AV, Zini A, Frayne R, Hill MD, Demchuk AM, Sajobi TT, Forkert ND, Goyal M, Lee TY, Menon BK. Time-Dependent Computed Tomographic Perfusion Thresholds for Patients With Acute Ischemic Stroke. Stroke 2015;46:3390-7. [Crossref] [PubMed]
- Ribo M, Molina CA, Cobo E, Cerdà N, Tomasello A, Quesada H, De Miquel MA, Millan M, Castaño C, Urra X, Sanroman L, Dàvalos A, Jovin TREVASCAT Trial Investigators. Association Between Time to Reperfusion and Outcome Is Primarily Driven by the Time From Imaging to Reperfusion. Stroke 2016;47:999-1004. [Crossref] [PubMed]
- Tsai JP, Mlynash M, Christensen S, Kemp S, Kim S, Mishra NK, Federau C, Nogueira RG, Jovin TG, Devlin TG, Akhtar N, Yavagal DR, Bammer R, Straka M, Zaharchuk G, Marks MP, Albers GW, Lansberg MG. CRISP Investigators. Time From Imaging to Endovascular Reperfusion Predicts Outcome in Acute Stroke. Stroke 2018;49:952-7. [Crossref] [PubMed]
- Yoo AJ, Andersson T. Thrombectomy in Acute Ischemic Stroke: Challenges to Procedural Success. J Stroke 2017;19:121-30. [Crossref] [PubMed]
- Sunde L, Vejerslev LO, Larsen JK, Christensen IJ, Hansen HE, Mogensen B, Bolund L. Genetically different cell subpopulations in hydatidiform moles. A study of three cases by RFLP, flow cytometric, cytogenetic, HLA, and morphologic analyses. Cancer Genet Cytogenet 1989;37:179-92. [Crossref] [PubMed]
- Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, Baumgart M, Leischner H, Schön G, Minnerup J, Thomalla G, Fiehler J, Kemmling A. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain 2019;142:1399-407. [Crossref] [PubMed]
- Broocks G, Fiehler J, Kemmling A. Collateral scoring in acute stroke patients with low ASPECTS: an unnecessary or underestimated tool for treatment selection? Brain 2019;142:e36. [Crossref] [PubMed]
- Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Michel P, Hajdu SD, Ribo M, Requena M, Maegerlein C, Friedrich B, Costalat V, Benali A, Pierot L, Gawlitza M, Schaafsma J, Mendes Pereira V, Gralla J, Fischer U. Mechanical Thrombectomy in Ischemic Stroke Patients With Alberta Stroke Program Early Computed Tomography Score 0-5. Stroke 2019;50:880-8. [Crossref] [PubMed]
- Panni P, Gory B, Xie Y, Consoli A, Desilles JP, Mazighi M, Labreuche J, Piotin M, Turjman F, Eker OF, Bracard S, Anxionnat R, Richard S, Hossu G, Blanc R, Lapergue B. ETIS (Endovascular Treatment in Ischemic Stroke) Investigators. Acute Stroke With Large Ischemic Core Treated by Thrombectomy. Stroke 2019;50:1164-71. [Crossref] [PubMed]
- Cagnazzo F, Derraz I, Dargazanli C, Lefevre PH, Gascou G, Riquelme C, Bonafe A, Costalat V. Mechanical thrombectomy in patients with acute ischemic stroke and ASPECTS ≤6: a meta-analysis. J Neurointerv Surg 2020;12:350-5. [Crossref] [PubMed]
- Hasegawa Y, Fisher M, Latour LL, Dardzinski BJ, Sotak CH. MRI diffusion mapping of reversible and irreversible ischemic injury in focal brain ischemia. Neurology 1994;44:1484-90. [Crossref] [PubMed]
- Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab 1995;15:1002-11. [Crossref] [PubMed]
- Lin W, Lee JM, Lee YZ, Vo KD, Pilgram T, Hsu CY. Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke 2003;34:64-70. [Crossref] [PubMed]
- Purushotham A, Campbell BC, Straka M, Mlynash M, Olivot JM, Bammer R, Kemp SM, Albers GW, Lansberg MG. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke 2015;10:348-53. [Crossref] [PubMed]
- Shinoda N, Hori S, Mikami K, Bando T, Shimo D, Kuroyama T, Kuramoto Y, Matsumoto M, Hirai O, Ueno Y. Utility of relative ADC ratio in patient selection for endovascular revascularization of large vessel occlusion. J Neuroradiol 2017;44:185-91. [Crossref] [PubMed]
- Scheldeman L, Wouters A, Bertels J, Dupont P, Cheng B, Ebinger M, Endres M, Fiebach JB, Gerloff C, Muir KW, Nighoghossian N, Pedraza S, Simonsen CZ, Thijs V, Thomalla G, Lemmens R. Reversibility of Diffusion-Weighted Imaging Lesions in Patients With Ischemic Stroke in the WAKE-UP Trial. Stroke 2023;54:1560-8. [Crossref] [PubMed]
- Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol 2004;55:207-12. [Crossref] [PubMed]
- Li F, Han SS, Tatlisumak T, Liu KF, Garcia JH, Sotak CH, Fisher M. Reversal of acute apparent diffusion coefficient abnormalities and delayed neuronal death following transient focal cerebral ischemia in rats. Ann Neurol 1999;46:333-42. [Crossref] [PubMed]
- Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, Eckert B, Wittkugel O, Weiller C, Zeumer H, Röther J. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke 2004;35:514-9. [Crossref] [PubMed]
- Luijten SP, Compagne KC, van Es AC, Roos YB, Majoie CB, van Oostenbrugge RJ, van Zwam WH, Dippel DW, Wolters FJ, van der Lugt A, Bos D. Brain atrophy and endovascular treatment effect in acute ischemic stroke: a secondary analysis of the MR CLEAN trial. Int J Stroke 2021; Epub ahead of print. [Crossref]
- Wáng YXJ. An explanation for the triphasic dependency of apparent diffusion coefficient (ADC) on T2 relaxation time: the multiple T2 compartments model. Quant Imaging Med Surg 2025;15:3779-91. [Crossref] [PubMed]
- Wáng YXJ, Ma FZ. A tri-phasic relationship between T2 relaxation time and magnetic resonance imaging (MRI)-derived apparent diffusion coefficient (ADC). Quant Imaging Med Surg 2023;13:8873-80. [Crossref] [PubMed]
- Wáng YXJ. Natural course of apparent diffusion coefficient (ADC) change after brain ischemic stroke: an alternative explanation by the triphasic relationship between T2 and ADC. Quant Imaging Med Surg 2024;14:9848-55. [Crossref] [PubMed]
- Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J. Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg 2017;9:117-21. [Crossref] [PubMed]
- Kaesmacher J, Dobrocky T, Heldner MR, Bellwald S, Mosimann PJ, Mordasini P, Bigi S, Arnold M, Gralla J, Fischer U. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J Neurol Neurosurg Psychiatry 2018;89:910-7. [Crossref] [PubMed]
- Chamorro Á, Blasco J, López A, Amaro S, Román LS, Llull L, Renú A, Rudilosso S, Laredo C, Obach V, Urra X, Planas AM, Leira EC, Macho J. Complete reperfusion is required for maximal benefits of mechanical thrombectomy in stroke patients. Sci Rep 2017;7:11636. [Crossref] [PubMed]
- Eiser AR. Cross-cultural perspectives on violence: is violence always violence? Mt Sinai J Med 1996;63:87-9.
- Dargazanli C, Fahed R, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, Saleme S, Costalat V, Bracard S, Desal H, Mazighi M, Consoli A, Piotin M, Lapergue BASTER Trial Investigators. Modified Thrombolysis in Cerebral Infarction 2C/Thrombolysis in Cerebral Infarction 3 Reperfusion Should Be the Aim of Mechanical Thrombectomy: Insights From the ASTER Trial (Contact Aspiration Versus Stent Retriever for Successful Revascularization). Stroke 2018;49:1189-96. [Crossref] [PubMed]
- Gilgen MD, Klimek D, Liesirova KT, Meisterernst J, Klinger-Gratz PP, Schroth G, Mordasini P, Hsieh K, Slotboom J, Heldner MR, Broeg-Morvay A, Mono ML, Fischer U, Mattle HP, Arnold M, Gralla J, El-Koussy M, Jung S. Younger Stroke Patients With Large Pretreatment Diffusion-Weighted Imaging Lesions May Benefit From Endovascular Treatment. Stroke 2015;46:2510-6. [Crossref] [PubMed]
- Donnan GA, Davis SM. Breaking the 3 h barrier for treatment of acute ischaemic stroke. Lancet Neurol 2008;7:981-2. [Crossref] [PubMed]
- Dávalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM, Silva Y, Serena J, Castillo J. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004;62:2187-92. [Crossref] [PubMed]
- Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, Kemp S, Albers GW. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke 2008;39:2491-6. [Crossref] [PubMed]
- Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med 2019;380:1795-803. [Crossref] [PubMed]
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med 2018;379:611-22. [Crossref] [PubMed]



