
Quantification of normal skin thickness using very high-frequency ultrasound: a clinical study in Chinese adults
Introduction
High-frequency ultrasound (HFUS), defined as ultrasound with a probe frequency exceeding 10 MHz, offers enhanced imaging capabilities. Probes operating above 20 MHz are termed very high-frequency ultrasound (VHFUS) (1,2). Conventional ultrasound achieves a resolution of 0.2 to 0.5 mm; inadequate for detailed skin assessments, as typical skin thickness ranges from 0.5 to 4 mm. HFUS enhances the resolution to 16 to 158 µm, enabling detailed visualization of the epidermis and dermis (3-5). VHFUS, with frequencies over 20 MHz, attains resolutions between 0.05 and 0.2 mm, sufficient for diagnosing skin pathologies. Moreover, 50-MHz probes surpass this, offering 0.1-mm resolution to distinctly reveal the hierarchical organization of the skin, particularly the superficial epidermis (3,5). As the ultrasound frequency increases, the resolution improves, but the penetration gradually decreases. Probes below 15 MHz are generally not recommended for skin lesion examinations because of their inadequate resolution (3,6-8). Various imaging techniques are currently used for skin assessment, such as digital photography, dermoscopy, optical coherence tomography, and multispectral optoacoustic tomography. Digital photography uses a digital camera to image the skin surface and is suitable for whole-body skin examination; however, it cannot provide information regarding the deep skin, and the cost of equipment upgrade is high. Dermoscopy can enlarge the skin surface image to observe the epidermis and superficial dermal papilla layer, which can be used to identify skin cancer; however, its utility for deep lesions is limited. Optical coherence tomography uses optical technology to obtain high-resolution images of the skin layer, which can provide images of the microstructure of the skin, but its equipment is expensive. Multispectral optoacoustic tomography is still in the research stage and is not widely used. HFUS and VHFUS can display all levels of the skin, provide deep skin information, evaluate the thickness of the skin and the boundary of the lesion, and image in real-time and at a lower cost. The resolution of VHFUS is higher than that of HFUS, and it can show the hierarchical structure of skin more clearly (9).
The skin serves as the primary defense barrier and protects the body against external damage. Ultrasound imaging typically reveals a superficial linear hyperechoic epidermis. Beneath this is the slightly hyperechoic dermal layer, with the hypoechogenic subcutaneous layer forming the deepest section. The dermal layer includes the thinner dermal papilla layer, located on the shallow surface, and the thicker dermal reticular layer, located deeper. The echo band of the dermal papillary layer may be lower than that of the dermal reticular layer; however, ultrasound cannot easily distinguish these two parts (10-12).
Skin thickness is a vital physiological indicator for monitoring various skin conditions, such as psoriasis, atopic dermatitis (AD), scleroderma, acne, and melanoma. These diseases may alter skin thickness differently across various stages and affected areas, highlighting the importance of precise measurements and imaging techniques, such as HFUS in dermatological evaluations. In 1979, Alexander and Miller first used HFUS to measure human skin thickness (13). Several studies have shown that HFUS can be used to measure skin thickness, which plays a particularly important role in the study of normal human skin and diagnosis and treatment of skin diseases (14-21). The purpose of this study was to compare differences in skin thickness measured by VHFUS and HFUS in healthy adults while also analyzing factors, such as sex, age, and body mass index (BMI) that may influence skin thickness measurements using VHFUS. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2637/rc).
Methods
Patient population
A total of 74 healthy volunteers were recruited from the Department of Ultrasound, West China Hospital of Sichuan University, between November 2022 and November 2023. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of West China Hospital of Sichuan University [approval No. 2023(488)]. Informed consent was obtained from all volunteers. The volunteers’ basic information, including sex, age, height, and weight, was recorded. The inclusion criteria were as follows: (I) age >18 years; (II) uniform skin color, no dandruff, no abnormal pigmentation, no space-occupying or other abnormal skin changes; (III) good inspection compliance. The exclusion criteria were as follows: (I) concomitant systemic or skin diseases; (II) pregnancy; (III) recent use of glucocorticoids or immunosuppressive agents; and (IV) poor compliance.
Examination site and body position
Inspection site: in accordance with the research of Nedelec et al. (14) and Moore et al. (22), the bilateral forearm (dorsal), upper arm (near the middle of triceps brachii), thigh (extension side median), calf (extension side median), dorsum of foot (middle), forehead (median), chest wall (between sternal angle and sternal notch), abdominal wall (subxiphoid) and back (lower part) skin were selected for measurement (Figure 1).
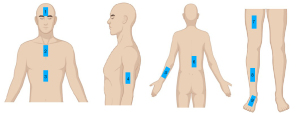
VHFUS and HFUS examination
The device used for examination was the Maida MD-300 SⅡ Skin Ultrasound Bio microscope (Tianjin Maida Medical Technology Co. Ltd., Tianjin, China) operating with 20 and 50 MHz probes. A 50 MHz probe was employed to obtain both transverse and longitudinal ultrasonographic images of various skin areas. The 20 MHz probe was used to acquire ultrasonographic images of the transverse and longitudinal sections of the skin on the chest and abdomen. To obtain normal skin ultrasound images, a thick layer of ultrasound gel was placed on the surface, and the probe was positioned perpendicular to the skin surface without applying pressure. Each skin area was first scanned longitudinally and then transversely to obtain normal ultrasound images of the skin. Ultrasound imaging revealed a normal skin structure characterized by two parallel high-echo bands separated by a wide low-echo zone. The shallowest high-echo band represented the epidermis, whereas the deeper high-echo band corresponded to the reflected echo at the dermis-subcutaneous tissue junction. The middle low-echo zone included the dermal papilla and reticular layers, which exhibited slightly higher echo levels. The subcutaneous layer exhibited the lowest echo because of its fat content (Figure 2). The obtained ultrasound images depicted clearly delineated and parallel double-layered linear hyperechoic structures with a uniformly applied coupling agent to prevent the formation of internal bubbles that could introduce artifacts and affect the measurement results. Epidermal, dermal, and full-skin thicknesses were measured separately, with three measurements taken and averaged.
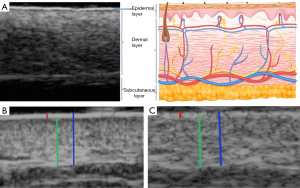
Ultrasound scanning was performed by two radiologists with 8 and 10 years of experience in superficial tissue ultrasound imaging. Imaging experts were blinded to the participants’ data. Before the study, the investigators agreed on the VHFUS and HFUS scanning and measurement techniques. A diagnostic consistency test was conducted, and the κ values of 0.884–0.999 were obtained. Briefly, the epidermal, dermal, and full skin thicknesses at the 14 sites were measured by two radiologists (Table 1).
Table 1
| Skin layer | ICC value (95% CI) | P |
|---|---|---|
| Epidermis | 0.843 (0.474, 0.951) | <0.01 |
| Dermis | 0.999 (0.997, 1.000) | <0.01 |
| Full skin | 0.999 (0.996, 1.000) | <0.01 |
CI, confidence interval; ICC, intraclass correlation coefficient.
Statistical analysis
Statistical analyses were performed using SPSS Statistics (v23.0; IBM Corp., Armonk, NY, USA). The statistical descriptions of measurement data with normal distributions are given as mean ± standard deviation. The statistical descriptions of measurement data with non-normal distributions are given as median (first quartile to third quartile). Independent-samples t-tests were used for comparisons between two independent samples. Paired samples were analyzed using a paired t-test, and comparisons between multiple groups of samples were performed using one-way analysis of variance. Pearson’s test was used for correlation analysis. Non-metric data were statistically described by frequency. The Chi-squared test was used to compare the rates or composition ratios of two or more groups. P<0.05 indicated that the difference was statistically significant.
Results
Patient characteristics
A total of 74 healthy volunteers were included in this study, including 41 men and 33 women; the ages ranged from 18 to 74 years, with an average age of 35 (interquartile range, 23.00–52.25) years. There was no significant difference in the BMI between volunteers of different sexes. There were significant differences in age between volunteers of different sexes (Table 2).
Table 2
| Characteristics | Numbers | Age (years) | BMI (kg/m2) | |||||
|---|---|---|---|---|---|---|---|---|
| Range | Median (IQR) | P value | Range | Mean ± SD | P value | |||
| All volunteers | 74 | 18–74 | 35.00 (23.00–52.25) | – | 17.01–33.79 | 25.51±3.55 | – | |
| Gender | <0.05 | 0.110 | ||||||
| Male | 41 | 18–66 | 30.00 (22.00–47.50) | 17.10–33.79 | 24.10±3.53 | |||
| Female | 33 | 21–74 | 45.00 (26.00–54.00) | 17.01–32.83 | 22.77±3.49 | |||
BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Comparison of skin thickness measured by VHFUS and HFUS
Comparison of longitudinal sections
The abdominal and chest epidermis thicknesses measured using VHFUS were lower than those measured using HFUS (P<0.05). There was no significant difference in the thickness of the dermis and full-thickness skin of the abdominal and chest walls as measured using VHFUS and HFUS (all P>0.05).
Comparison of transverse sections
The thickness of the abdomen and chest epidermis measured using VHFUS was less than that measured using HFUS (P<0.05). There was no significant difference in the thickness of the dermis and full-thickness skin of the abdomen and chest measured using VHFUS and HFUS (all P>0.05).
Comparison of skin thickness measured by VHFUS in different sections
There was no significant difference in the thickness of the epidermis, dermis, or full thickness of the skin between the transverse and longitudinal sections (all P>0.05; Table 3).
Table 3
| Parts | Thickness of epidermis (mm) | Thickness of dermis (mm) | Full thickness of skin (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longitudinal | Transverse | t | P | Longitudinal | Transverse | t | P | Longitudinal | Transverse | t | P | |||
| Abdominal | 0.1100±0.0178 | 0.1097±0.0156 | 0.139 | 0.909 | 2.3919±0.5431 | 2.4243±0.5549 | −0.359 | 0.720 | 2.5130±0.5383 | 2.5347±0.5527 | −0.242 | 0.809 | ||
| Chest | 0.1039±0.0190 | 0.1038±0.0171 | 0.003 | 0.976 | 1.9559±0.5035 | 1.9831±0.5067 | −0.328 | 0.744 | 2.0689±0.5039 | 2.0961±0.5065 | −0.327 | 0.744 | ||
| Forehead | 0.0990±0.0185 | 0.0992±0.0176 | −0.076 | 0.940 | 2.1545±0.5422 | 2.1595±0.5351 | −0.056 | 0.955 | 2.2645±0.5431 | 2.2736±0.5418 | −0.103 | 0.918 | ||
| Right forearm | 0.1178±0.0185 | 0.1132±0.0189 | 1.496 | 0.137 | 1.6138±0.4217 | 1.6360±0.4275 | −0.319 | 0.750 | 1.7324±0.4311 | 1.7497±0.4217 | −0.246 | 0.806 | ||
| Right upper arm | 0.1129±0.0170 | 0.1130±0.0218 | −0.014 | 0.989 | 1.6139±0.4353 | 1.5949±0.4254 | 0.268 | 0.789 | 1.7319±0.4329 | 1.7107±0.4244 | 0.300 | 0.764 | ||
| Left forearm | 0.1146±0.0165 | 0.1169±0.0345 | −0.527 | 0.599 | 1.5696±0.3900 | 1.5729±0.4061 | −0.052 | 0.959 | 1.6772±0.3898 | 1.6902±0.4070 | −0.199 | 0.843 | ||
| Left upper arm | 0.1167±0.0180 | 0.1134±0.0179 | 1.116 | 0.266 | 1.6681±0.5227 | 1.6916±0.5076 | −0.278 | 0.781 | 1.7820±0.5220 | 1.8081±0.5088 | −0.308 | 0.758 | ||
| Back | 0.1184±0.0189 | 0.1172±0.0194 | 0.372 | 0.710 | 2.9167±0.6262 | 2.8967±0.6029 | 0.197 | 0.844 | 3.0363±0.6269 | 3.0146±0.6113 | 0.213 | 0.832 | ||
| Right thigh | 0.1166±0.0168 | 0.1158±0.0155 | 0.322 | 0.748 | 1.7715±0.5150 | 1.7745±0.5272 | −0.035 | 0.972 | 1.8823±0.5161 | 1.8868±0.5290 | −0.052 | 0.959 | ||
| Right leg | 0.1128±0.0161 | 0.1124±0.0181 | 0.144 | 0.886 | 1.7977±0.4987 | 1.7843±0.4717 | 0.168 | 0.867 | 1.8964±0.4827 | 1.9128±0.4872 | −0.207 | 0.836 | ||
| Right foot dorsum | 0.1147±0.0227 | 0.1163±0.0238 | −0.401 | 0.689 | 1.1491±0.2850 | 1.1410±0.2739 | 0.175 | 0.861 | 1.2626±0.2838 | 1.2579±0.2797 | 0.100 | 0.920 | ||
| Left thigh | 0.1151±0.0172 | 0.1150±0.0153 | 0.052 | 0.958 | 1.7120±0.4880 | 1.7410±0.5220 | −0.349 | 0.728 | 1.8368±0.4915 | 1.8498±0.5203 | −0.156 | 0.876 | ||
| Left leg | 0.1129±0.0169 | 0.1113±0.0170 | 0.598 | 0.551 | 1.7164±0.4342 | 1.7396±0.4102 | −0.333 | 0.740 | 1.8336±0.4330 | 1.8491±0.4114 | −0.224 | 0.823 | ||
| Left foot dorsum | 0.1135±0.0196 | 0.1147±0.0186 | −0.401 | 0.689 | 1.1389±0.2536 | 1.1445±0.2733 | −0.138 | 0.890 | 1.2475±0.2526 | 1.2607±0.2781 | −0.302 | 0.763 | ||
Data are presented as mean ± standard deviation.
Comparison of the left and right sides of the skin thickness measured by VHFUS
Because there was no statistically significant difference between the transverse and longitudinal sections and the longitudinal section had a better fit with the skin, we selected the longitudinal section of the VHFUS measurement to analyze the influencing factors of skin thickness.
There was no significant difference in the epidermis thickness between the left and right sides of the forearm, upper arm, thigh, calf, or back of the foot (all P>0.05).
The dermis and full-thickness skin of the right forearm and calf were thicker than those of the left side (all P<0.05). There were no significant differences in the remaining areas (all P<0.05; Figure 3).
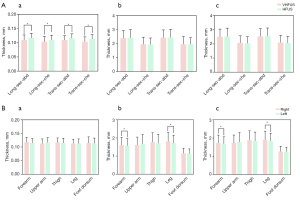
Comparison of skin thickness measured by VHFUS in different sexes
As there was no difference in the thickness of the epidermis between the two sides, only the thickness of the forearm and calf differed between the dermis and the full thickness of the skin. Therefore, in addition to the forearm and calf, we chose the right skin thickness measurement for statistical analysis.
There was no significant difference in the epidermis thickness between sexes (all P>0.05). Except for the back, the thicknesses of the dermis and full-thickness skin were greater in males than in females. The differences in the right forearm, right upper arm, left forearm, right thigh, right calf, and left calf were statistically significant (all P<0.05; Table 4).
Table 4
| Parts | Thickness of epidermis (mm) | Thickness of dermis (mm) | Full thickness of skin (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | t | P | Male | Female | t | P | Male | Female | t | P | |||
| Abdominal | 0.1102±0.0184 | 0.1097±0.0173 | 0.131 | 0.896 | 2.4803±0.5553 | 2.2820±0.5148 | 1.577 | 0.119 | 2.5951±0.5567 | 2.4110±0.5042 | 1.474 | 0.145 | ||
| Chest | 0.1024±0.0189 | 0.1057±0.0191 | −0.724 | 0.472 | 2.0430±0.4754 | 1.8478±0.5237 | 1.678 | 0.098 | 2.1548±0.4762 | 1.9623±0.5240 | 1.653 | 0.103 | ||
| Forehead | 0.1010±0.0205 | 0.0965±0.0157 | 1.041 | 0.301 | 2.1990±0.5019 | 2.0991±0.5917 | 0.785 | 0.435 | 2.3092±0.5038 | 2.2089±0.5915 | 0.788 | 0.434 | ||
| Right forearm | 0.1159±0.0187 | 0.1201±0.0183 | −0.963 | 0.339 | 1.7738±0.4099 | 1.4149±0.3495 | 3.994 | <0.05 | 1.8967±0.4220 | 1.5283±0.3519 | 4.015 | <0.05 | ||
| Right upper arm | 0.1118±0.0162 | 0.1144±0.0181 | −0.666 | 0.508 | 1.7820±0.3765 | 1.4051±0.4170 | 4.080 | <0.05 | 1.8972±0.3793 | 1.5266±0.4112 | 4.024 | <0.05 | ||
| Left forearm | 0.1126±0.0152 | 0.1170±0.0180 | −1.133 | 0.261 | 1.6953±0.3924 | 1.4133±0.3304 | 3.293 | <0.05 | 1.8005±0.3936 | 1.5239±0.3305 | 3.223 | <0.05 | ||
| Back | 0.1182±0.0200 | 0.1186±0.0178 | −0.084 | 0.933 | 2.9069±0.6708 | 2.9288±0.5761 | −0.148 | 0.882 | 3.0159±0.6630 | 3.0616±0.5881 | −0.310 | 0.758 | ||
| Right thigh | 0.1148±0.0146 | 0.1189±0.0193 | −1.041 | 0.301 | 1.9380±0.5463 | 1.5645±0.3902 | 3.305 | <0.05 | 2.0524±0.5446 | 1.6710±0.3922 | 3.377 | <0.05 | ||
| Right leg | 0.1128±0.0153 | 0.1128±0.0174 | −0.017 | 0.987 | 1.9676±0.5118 | 1.5867±0.3965 | 3.510 | <0.05 | 2.0522±0.4931 | 1.7027±0.3972 | 3.299 | <0.05 | ||
| Left leg | 0.1120±0.0145 | 0.1140±0.0196 | −0.507 | 0.614 | 1.8510±0.4521 | 1.5493±0.3501 | 3.147 | <0.05 | 1.9690±0.4493 | 1.6653±0.3502 | 3.182 | <0.05 | ||
| Right foot dorsum | 0.1150±0.0227 | 0.1143±0.0229 | 0.131 | 0.896 | 1.2310±0.2987 | 1.0475±0.2338 | 2.887 | 0.050 | 1.3391±0.2966 | 1.1674±0.2385 | 2.695 | 0.090 | ||
Data are presented as mean ± standard deviation.
Correlation analysis of skin thickness with age and BMI
The correlation analysis between skin thickness and age revealed that the thickness of the epidermis on the dorsal side of the right foot, the dermis of the right leg, and the total skin thickness exhibited negative correlations with age (all P<0.05). In contrast, no significant correlations were observed between skin thickness and age in the other examined regions (all P>0.05, Table 5).
Table 5
| Parts | Epidermis | Dermis | Full skin | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |||
| Abdominal | −0.084 | 0.477 | −0.066 | 0.574 | −0.064 | 0.586 | ||
| Chest | −0.107 | 0.365 | −0.032 | 0.789 | −0.026 | 0.829 | ||
| Forehead | −0.210 | 0.073 | −0.151 | 0.199 | −0.149 | 0.206 | ||
| Right forearm | −0.016 | 0.894 | −0.028 | 0.812 | −0.041 | 0.731 | ||
| Right upper arm | −0.031 | 0.792 | 0.017 | 0.886 | 0.014 | 0.909 | ||
| Left forearm | 0.112 | 0.343 | 0.010 | 0.932 | 0.021 | 0.862 | ||
| Back | −0.165 | 0.160 | −0.036 | 0.764 | −0.060 | 0.613 | ||
| Right thigh | −0.052 | 0.657 | −0.172 | 0.143 | 0.175 | 0.137 | ||
| Right leg | −0.072 | 0.542 | −0.245 | <0.05 | −0.229 | <0.05 | ||
| Left leg | 0.021 | 0.860 | −0.178 | 0.130 | −0.187 | 0.111 | ||
| Right foot dorsum | −0.257 | <0.05 | 0.012 | 0.922 | 0.029 | 0.809 | ||
Correlation analysis between skin thickness and BMI showed that the thicknesses of the chest wall, right forearm, right upper arm, left forearm, right upper arm, right foot dorsal dermis, and full thickness of the skin were positively correlated with BMI (all P<0.05). In contrast, the others showed no significant correlation between skin thickness and BMI (Table 6).
Table 6
| Parts | Epidermis | Dermis | Full skin | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |||
| Abdominal | 0.070 | 0.554 | 0.183 | 0.118 | 0.179 | 0.128 | ||
| Chest | 0.112 | 0.342 | 0.302 | <0.05 | 0.306 | <0.05 | ||
| Forehead | 0.066 | 0.578 | 0.065 | 0.583 | 0.065 | 0.582 | ||
| Right forearm | 0.163 | 0.166 | 0.313 | <0.05 | 0.314 | <0.05 | ||
| Right upper arm | 0.101 | 0.394 | 0.300 | <0.05 | 0.291 | <0.05 | ||
| Left forearm | −0.075 | 0.525 | 0.299 | <0.05 | 0.302 | <0.05 | ||
| Back | 0.020 | 0.867 | 0.106 | 0.368 | 0.090 | 0.445 | ||
| Right thigh | 0.104 | 0.380 | 0.263 | <0.05 | 0.262 | <0.05 | ||
| Right leg | 0.051 | 0.669 | 0.096 | 0.416 | 0.095 | 0.423 | ||
| Left leg | 0.116 | 0.325 | 0.096 | 0.414 | 0.095 | 0.419 | ||
| Right foot dorsum | −0.200 | 0.088 | 0.255 | <0.05 | 0.257 | <0.05 | ||
BMI, body mass index.
Comparison of skin thickness measured by VHFUS in different parts
The epidermis of 11 healthy volunteers, from thin to thick, was as follows: forehead, chest, abdomen, right calf, left calf, upper arm, left forearm, foot back, thigh, back, and right forearm. The differences in epidermal thickness between the different parts were statistically significant (all P<0.05).
The dermis in 11 healthy volunteers, from thin to thick, was as follows: foot back, left forearm, right forearm, upper arm, left calf, thigh, right calf, chest, forehead, abdomen, and back. The differences in dermal thickness between the different parts were statistically significant (all P<0.05).
The full-thickness skin thickness of the 11 healthy volunteers, from thin to thick, was as follows: foot back, left forearm, upper arm, right forearm, left calf, thigh, right calf, chest, forehead, abdomen, and back. The difference in full-thickness skin thickness between the different parts was statistically significant (all P<0.05; Figure 4).

Discussion
The skin is structurally divided into the epidermis and dermis. Skin thickness is a common physiological measurement indicator, and many skin diseases are often accompanied by changes in skin thickness. Monitoring skin thickness in patients can effectively assess the degree of skin disease lesions, providing a quantitative reference for the treatment of skin diseases. AD is a common inflammatory skin disease, clinically manifested by recurrent eczema, dry skin, itching, and thickening. This disease has significant social and psychological impacts on patients and their families. Currently, the diagnosis of AD mainly relies on clinical features (23). Sorokina et al. (24) used 75 MHz VHFUS to collect skin sonograms from 22 children with AD aged 0–8 years and 18 healthy children. They found that 59% of patients with AD had irregular epidermal contours, with a trend of epidermal thickening and dermal thinning. Additionally, the presence of subepidermal low-echogenic bands (SLEBs) was observed in 77% of cases, providing a reference for the diagnosis of AD. Liu et al. (18) used VHFUS to measure the thickness of SLEB as a quantitative indicator, evaluating the therapeutic effect of dupilumab on severe AD. The results indicated that VHFUS not only allowed dynamic observation of skin changes in patients with AD patients, providing visible evidence for diagnosing AD, but also enabled monitoring of skin changes after drug treatment, assessing the therapeutic effect. Dini et al. (25) used VHFUS to assess changes in the epidermis and SLEB in patients with AD after dupilumab treatment and compared them with changes after conventional treatment, evaluating the efficacy of dupilumab. Systemic sclerosis (SSc) is a chronic connective tissue disease affecting multiple organs, leading to multiple organ failure and high mortality. Timely diagnosis and determination of the active phase of the disease are challenging. Currently, the primary diagnostic method for SSc is the modified Rodnan skin score (mRSS) (26). Hesselstrand et al. (27) conducted HFUS on 75 patients with SSc with disease duration of less than 3 years and followed them for 1 year. The study focused on early changes in skin thickness in patients with SSc and found that shorter disease duration was associated with thicker skin. Yang et al. (28) included 49 patients with limited cutaneous systemic sclerosis (lcSSc) and 51 patients with diffuse cutaneous systemic sclerosis (dcSSc), and measured skin thickness using HFUS. The results showed that, except for the fingers, the skin thickness in most areas of dcSSc was thicker than that in lcSSc (P<0.05). In lcSSc, due to the longer disease duration, the skin thickness in the forearm, upper arm, chest wall, abdomen, and thigh were thinner. HFUS can be used to observe skin thickness and provide quantitative and objective evaluation for early SSc.
VHFUS and HFUS have also been applied in the research of other diseases. Dini et al. (29) used a 70-MHz probe to evaluate changes in plaque psoriasis before and after ixekizumab treatment. The results showed that, after treatment, the thickness of the surface hyperechoic bands gradually thinned, and the thickness of SLEB also decreased. VHFUS can provide accurate reference values that are useful for assessing disease changes. Russo et al. (30) used HFUS and VHFUS to examine 88 patients with suspected primary cutaneous lymphoma. They categorized the lesions, and pathology was used to confirm the ultrasound findings. The final results indicated that VHFUS offered valuable insights into the imaging characteristics of primary cutaneous lymphomas, aiding in accurate diagnosis and assessment of treatment response. Michelucci et al. (31) showed that VHFUS significantly improved the detection of microtunnels in hidradenitis suppurativa, providing a reference for timely intervention and preventing disease progression. VHFUS is increasingly being applied in the field of dermatology.
Comparison of skin thickness measured by VHFUS and HFUS
This study revealed a significant difference in epidermal thickness between the abdominal and chest areas using VHFUS (50 MHz) and HFUS (20 MHz). The resolution of HFUS can reach 0.016–0.158 mm, which can clearly show the epidermis and dermis. VHFUS offers a resolution finer than 0.1 mm, which is sufficient for diagnosing skin pathologies (3,5). VHFUS demonstrates higher frequency, superior resolution, and a clearer display of the epidermis. Measurements of epidermal thickness obtained using VHFUS were more precise than those obtained using HFUS, indicating that VHFUS is a suitable modality for assessing epidermal thickness. In contrast, no significant differences were observed in the measured dermal and overall skin values between VHFUS and HFUS. The reason for this may be that the dermis and the whole skin are thicker than the epidermis, and the difference of thickness is small. These differences could not be compared between VHFUS and HFUS. This may suggest that when measuring the dermis and overall skin thickness, both the 20 and 50 MHz probes performed similarly in terms of accuracy and reliability, providing consistent results. Nevertheless, owing to its enhanced imaging capabilities, VHFUS may be preferred for dermal thickness measurements, such as in port wine stains showing thickening of the skin. HFUS can be used to measure the thickness of skin with lesions and compare it with the thickness of healthy skin (32); however, VHFUS uses a higher frequency, improving the clarity and accuracy of the skin display and reducing the measurement error. In this way, differences between the lesion and the healthy skin can be more easily identified. The increased resolution offered by the 50-MHz probe is especially beneficial when evaluating fine structures or thin layers within the skin, where even minor differences in thickness are significant. It is important to acknowledge, however, that VHFUS has limited penetration, with a maximum depth of 5 mm. Consequently, the measurement of lesions with a thickened dermis may be subject to inaccuracies owing to restricted penetration. To address these limitations, high-frequency probes using slightly lower frequencies can be used to measure dermal thickness more effectively (9).
Influence of scanning section on the measured value of skin thickness
In this study, VHFUS was used to measure the thicknesses of the epidermis, dermis, and overall skin in various sections (transverse and longitudinal) at the same anatomical site. The results indicated no significant difference in the thickness of the epidermis, dermis, or overall skin obtained from different sections of the same site. Consequently, the skin thickness can be accurately assessed by selecting the most appropriate section based on the specific inspection site. For instance, owing to the convex shape of the skull, the longitudinal section of the forehead skin provides a better fit than the transverse section, thereby minimizing measurement errors associated with poor probe-skin contact. Our findings suggest that if a particular surface is unsuitable for measurement for any reason, an alternative section should be utilized for inspection and assessment.
Bilateral comparison of skin thickness
There was no significant difference in the thickness of the epidermis between the bilateral forearms, upper arms, thighs, calves, and dorsum of the feet. This finding suggests that the contralateral side can serve as a viable reference for comparative evaluation of unilateral epidermal lesions. However, the thicknesses of the bilateral forearms, calf dermis, and full-thickness skin were greater on the right side than on the left side. This discrepancy may be attributed to factors such as the volunteer’s working style, sun exposure, or dominance of the hand or foot. However, we did not investigate its potential influence on skin thickness. Future research should explore these factors in greater detail.
Comparison of skin thickness between different sexes
In this study, the epidermal thickness in women was slightly greater than that in men, except for the forehead and back of the right foot; however, this difference was not statistically significant. Conversely, men exhibited greater dermal and overall skin thicknesses than women, except for the back. Statistically significant differences were observed in the thickness of the dermis and full skin thickness of the right forearm, right upper arm, left forearm, right thigh, right calf, and left calf. Their findings indicated that males had greater dermal thickness than females, but only a few sites reached statistical significance. Similarly, Firooz et al. (33) employed both 22 and 50 MHz ultrasound to measure epidermal and dermal thicknesses in the cheek, neck, palm, dorsum of the foot, and sole of the foot among healthy individuals, revealing that while males had higher epidermal and dermal thicknesses, only the neck and dorsum of the foot showed statistically significant differences. Meng et al. (34) utilized 24 MHz ultrasound to assess the epidermal and dermal thicknesses of the forehead and glabella, among other regions, in a cohort of normal individuals. Their findings revealed that the thickness of both the epidermis and dermis in males was significantly greater than that in females, with statistically significant differences identified. Finally, Wang et al. (16) employed 22 or 75 MHz ultrasound to investigate epidermal and dermal thicknesses in 13 different anatomical sites in healthy individuals, such as the forehead, cheek, and abdomen. Their findings indicated that males had greater dermal thickness than females, but only a few sites reached statistical significance. Our study also found that the thickness of the dermis and the full-thickness skin were greater in men than in women except for on the back, with some areas showing statistically significant differences and no significant differences in the epidermis. In future studies, sample sizes should be appropriately increased to further study the difference in skin thickness between sexes.
Correlation analysis between age, BMI, and skin thickness
In this study, the thicknesses of the dorsal epidermis of the right foot, the dermis of the right leg, and the overall skin thickness were negatively correlated with age. Meng et al. (34) investigated the relationship between facial skin thickness and age using HFUS and found that the thicknesses of the zygomatic and submandibular dermis were significantly greater in young women than in middle-aged and elderly women (P<0.05). They observed a decrease in facial skin thickness in women of advanced age. In men, the correlation between epidermal and dermal thicknesses and age was significant only in the zygomatic dermis. Van Mulder et al. (35) employed HFUS to assess epidermal and dermal thicknesses at the proximal ventral and dorsal forearm and the deltoid region. Their findings indicated that age solely influenced skin thickness in the deltoid area. Nedelec et al. (14) also reported differences in skin thickness across various age groups. This study further indicates that certain skin areas exhibit a negative correlation with age. This may be related to the loss of skin collagen with age.
Several studies have shown that skin thickness may be related to factors such as BMI. Meng et al. (34) used HFUS to evaluate the relationship between facial skin thickness and sex, age, and BMI in healthy adults. The thicknesses of the cervical epidermis and dermis in women correlated with BMI, whereas there was no significant correlation between the thicknesses of the epidermis and dermis and BMI in men. Van Mulder et al. (35) found that the skin thickness of the proximal ventral, dorsal forearm, and deltoid muscles in healthy people was significantly correlated with BMI. We also found a weak positive correlation between the measured thickness of the dermal layer and the whole skin of the chest wall, right forearm, right upper arm, left forearm, right upper arm, right dorsum of the foot, and BMI (all P<0.05). However, it is important to note that skin thickness may be influenced by several factors, such as ethnicity, which could also have an impact on BMI. This relationship between BMI, ethnicity, and skin thickness was not explored in detail in our study, and the potential bias introduced by these factors might have contributed to the observed results. In future research, we plan to refine our understanding of the various factors that influence skin thickness.
Comparison of skin thickness in different parts
Many studies have shown differences in skin thickness between different parts (16,30,31). In the present study, there were statistically significant differences in the thickness of the epidermis, dermis, and full-thickness skin in 11 different areas in healthy volunteers (all P<0.05), consistent with previous research results. In this study, the thinnest part of the epidermis was 0.0990±0.0185 mm in the forehead, and the thickest part was 0.1184±0.0189 mm in the back; the thinnest part of the thickness of the dermis was the back of the foot 1.1491±0.2850 mm, and the thickest part was the back 2.9167±0.6262 mm; the thinnest part of the full-thickness skin thickness was 1.2626±0.2838 mm on the back of the foot, and the thickest part was 3.0363±0.6269 mm on the back. There are differences in the skin thickness in different parts of healthy individuals, which may be related to the different functions of the different parts.
The limitations of this study are as follows: (I) this study used a small sample, with very few participants over 70 years old or with a BMI less than 18.5 kg/m2, and the number of healthy volunteers should be expanded, especially in these groups; (II) differences in skin thickness measured by 50 and 20 MHz ultrasound in all parts were not studied.
Conclusions
In summary, VHFUS can obtain clear skin sonograms and accurately measure skin thickness. Compared to HFUS, VHFUS uses a higher frequency and has better resolution, providing a clearer display of the epidermal layer. Measurements of epidermal thickness obtained using VHFUS were more precise than those obtained using HFUS; there was no significant difference in skin thickness measured in transverse and longitudinal sections. The optimal section was selected for skin thickness measurement. For sites with no significant difference in skin thickness between the two sides, the contralateral side can be selected as a reference for unilateral lesions. Sex, age, and BMI have an impact on skin thickness measurements. Different parts of the skin have different thicknesses. It is necessary to establish corresponding reference ranges for normal skin thickness based on these influencing factors. This would be helpful for the diagnosis and evaluation of treatment effectiveness for skin diseases such as scleroderma, hemangioma, psoriasis, and AD. Future research should include factors that may affect skin thickness, such as different ethnicities, varying sun exposure times, and different occupations. Additionally, larger sample sizes are needed, especially of volunteers with low BMI (<18.5 kg/m2) and older age (>70 years), to further explore the factors influencing skin thickness.
Acknowledgments
Figure 1 was from Biorender (https://www.biorender.com), and we purchased the copyright for the images on the website. Figure 2A was commissioned and paid for by the company, the name of the commissioned company is Shenyang Mitang Graphic Technology Co., Ltd. Figure 2B,2C was self-retained skin ultrasonic sonogram by the authors. Figures 3,4 were created using GraphPad plotting software.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2637/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2637/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of West China Hospital of Sichuan University [approval No. 2023(488)]. Informed consent was obtained from all volunteers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Izzetti R, Oranges T, Janowska A, Gabriele M, Graziani F, Romanelli M. The Application of Ultra-High-Frequency Ultrasound in Dermatology and Wound Management. Int J Low Extrem Wounds 2020;19:334-40. [Crossref] [PubMed]
- Polańska A, Dańczak-Pazdrowska A, Jałowska M, Żaba R, Adamski Z. Current applications of high-frequency ultrasonography in dermatology. Postepy Dermatol Alergol 2017;34:535-42. [Crossref] [PubMed]
- Schneider SL, Kohli I, Hamzavi IH, Council ML, Rossi AM, Ozog DM. Emerging imaging technologies in dermatology: Part I: Basic principles. J Am Acad Dermatol 2019;80:1114-20. [Crossref] [PubMed]
- Lintzeri DA, Karimian N, Blume-Peytavi U, Kottner J. Epidermal thickness in healthy humans: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2022;36:1191-200. [Crossref] [PubMed]
- Ud-Din S, Foden P, Stocking K, Mazhari M, Al-Habba S, Baguneid M, McGeorge D, Bayat A. Objective assessment of dermal fibrosis in cutaneous scarring, using optical coherence tomography, high-frequency ultrasound and immunohistomorphometry of human skin. Br J Dermatol 2019;181:722-32. [Crossref] [PubMed]
- Wortsman X, Carreño L, Ferreira-Wortsman C, Poniachik R, Pizarro K, Morales C, Calderon P, Castro A. Ultrasound Characteristics of the Hair Follicles and Tracts, Sebaceous Glands, Montgomery Glands, Apocrine Glands, and Arrector Pili Muscles. J Ultrasound Med 2019;38:1995-2004. [Crossref] [PubMed]
- Mlosek RK, Migda B, Migda M. High-frequency ultrasound in the 21(st) century. J Ultrason 2021;20:e233-41. [Crossref] [PubMed]
- Wortsman X, Alfageme F, Roustan G, Arias-Santiago S, Martorell A, Catalano O, Scotto di Santolo M, Zarchi K, Bouer M, Gonzalez C, Bard R, Mandava A, Gaitini DGuidelines for Performing Dermatologic Ultrasound Examinations by the DERMUS Group. J Ultrasound Med 2016;35:577-80. [Crossref] [PubMed]
- Schneider SL, Kohli I, Hamzavi IH, Council ML, Rossi AM, Ozog DM. Emerging imaging technologies in dermatology: Part II: Applications and limitations. J Am Acad Dermatol 2019;80:1121-31. [Crossref] [PubMed]
- Gould J. Superpowered skin. Nature 2018;563:S84-5. [Crossref] [PubMed]
- Cisoń H, Białynicki-Birula R. High-frequency ultrasonography: one of the modern imaging diagnostic methods in dermatology. Authors' own experience and review. Postepy Dermatol Alergol 2024;41:306-13. [Crossref] [PubMed]
- Catalano O, Wortsman X. Dermatology Ultrasound. Imaging Technique, Tips and Tricks, High-Resolution Anatomy. Ultrasound Q 2020;36:321-7. [Crossref] [PubMed]
- Alexander H, Miller DL. Determining skin thickness with pulsed ultra sound. J Invest Dermatol 1979;72:17-9. [Crossref] [PubMed]
- Nedelec B, Forget NJ, Hurtubise T, Cimino S, de Muszka F, Legault A, Liu WL, de Oliveira A, Calva V, Correa JA. Skin characteristics: normative data for elasticity, erythema, melanin, and thickness at 16 different anatomical locations. Skin Res Technol 2016;22:263-75. [Crossref] [PubMed]
- Bezugly A, Rembielak A. The use of high frequency skin ultrasound in non-melanoma skin cancer. J Contemp Brachytherapy 2021;13:483-91. [Crossref] [PubMed]
- Wang S, Yu RX, Fan W, Li CX, Fei WM, Li S, Zhou J, Hu R, Liu M, Xu F, Xu J, Cui Y. Detection of skin thickness and density in healthy Chinese people by using high-frequency ultrasound. Skin Res Technol 2023;29:e13219. [Crossref] [PubMed]
- Luo Y, Wang J, Gao Y, Wang Y, Zhang S, Liu Z, Zhu Q, Liu J. Value of high-frequency ultrasound in the treatment of moderate and severe acne vulgaris. Skin Res Technol 2022;28:833-9. [Crossref] [PubMed]
- Liu Z, Niu Z, Zhang D, Liu J, Zhu Q. Improve the dupilumab therapy evaluation with dermoscopy and high-frequency ultrasound in moderate-to-severe atopic dermatitis. Skin Res Technol 2023;29:e13260. [Crossref] [PubMed]
- Wang J, Luo Y, Liu J, Zhu Q, Wang Y, Jin H. High-frequency ultrasonography and scoring of acne at 20 and 50 MHz. J Eur Acad Dermatol Venereol 2020;34:e743-5. [Crossref] [PubMed]
- Wang YK, Gao YJ, Liu J, Zhu QL, Wang JC, Qin J, Jin HZ. A comparative study of melanocytic nevi classification with dermoscopy and high-frequency ultrasound. Skin Res Technol 2022;28:265-73. [Crossref] [PubMed]
- Oranges T, Janowska A, Scatena C, Faita F, Lascio ND, Izzetti R, Fidanzi C, Romanelli M, Dini V. Ultra-High Frequency Ultrasound in Melanoma Management: A New Combined Ultrasonographic-Histopathological Approach. J Ultrasound Med 2023;42:99-108. [Crossref] [PubMed]
- Moore TL, Lunt M, McManus B, Anderson ME, Herrick AL. Seventeen-point dermal ultrasound scoring system--a reliable measure of skin thickness in patients with systemic sclerosis. Rheumatology (Oxford) 2003;42:1559-63. [Crossref] [PubMed]
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020;396:345-60. [Crossref] [PubMed]
- Sorokina E, Mikailova D, Krakhaleva J, Krinitsyna J, Yakubovich A, Sergeeva I. Ultrasonography patterns of atopic dermatitis in children. Skin Res Technol 2020;26:482-8. [Crossref] [PubMed]
- Dini V, Iannone M, Michelucci A, Manzo Margiotta F, Granieri G, Salvia G, Oranges T, Janowska A, Morganti R, Romanelli M. Ultra-High Frequency UltraSound (UHFUS) Assessment of Barrier Function in Moderate-to-Severe Atopic Dermatitis during Dupilumab Treatment. Diagnostics (Basel) 2023;13:2721. [Crossref] [PubMed]
- Volkmann ER, Andréasson K, Smith V. Systemic sclerosis. Lancet 2023;401:304-18. [Crossref] [PubMed]
- Hesselstrand R, Carlestam J, Wildt M, Sandqvist G, Andréasson K. High frequency ultrasound of skin involvement in systemic sclerosis - a follow-up study. Arthritis Res Ther 2015;17:329. [Crossref] [PubMed]
- Yang Y, Tang X, Zhong L, Zhang L, Tang Y, Wang Y, Lv X, Qiu L. Shear wave elastography-based skin assessment system for systemic sclerosis: a supplement or alternative to conventional ultrasound? Quant Imaging Med Surg 2023;13:4405-14. [Crossref] [PubMed]
- Dini V, Janowska A, Faita F, Panduri S, Benincasa BB, Izzetti R, Romanelli M, Oranges T. Ultra-high-frequency ultrasound monitoring of plaque psoriasis during ixekizumab treatment. Skin Res Technol 2021;27:277-82. [Crossref] [PubMed]
- Russo A, Patanè V, Gagliardi F, Urraro F, Ronchi A, Vitiello P, Sica A, Argenziano G, Nardone V, Reginelli A. Preliminary Experience in Ultra-High Frequency Ultrasound Assessment of Cutaneous Primary Lymphomas: An Innovative Classification. Cancers (Basel) 2024;16:2456. [Crossref] [PubMed]
- Michelucci A, Granieri G, Cei B, Manzo Margiotta F, Janowska A, Oranges T, Romanelli M, Dini V. Enhancing Hidradenitis Suppurativa Assessment: The Role of Ultra-High Frequency Ultrasound in Detecting Microtunnels and Refining Disease Staging. J Ultrasound Med 2025;44:739-45. [Crossref] [PubMed]
- Tang Y, Cheng S, Tang X, Guo R, Zhang L, Qiu L. Quantification of skin lesions using high-frequency ultrasound and shear wave elastography in port-wine stain patients: a clinical study. Ann Transl Med 2019;7:803. [Crossref] [PubMed]
- Firooz A, Rajabi-Estarabadi A, Zartab H, Pazhohi N, Fanian F, Janani L. The influence of gender and age on the thickness and echo-density of skin. Skin Res Technol 2017;23:13-20. [Crossref] [PubMed]
- Meng Y, Feng L, Shan J, Yuan Z, Jin L. Application of high-frequency ultrasound to assess facial skin thickness in association with gender, age, and BMI in healthy adults. BMC Med Imaging 2022;22:113. [Crossref] [PubMed]
- Van Mulder TJ, de Koeijer M, Theeten H, Willems D, Van Damme P, Demolder M, De Meyer G, Beyers KC, Vankerckhoven V. High frequency ultrasound to assess skin thickness in healthy adults. Vaccine 2017;35:1810-5. [Crossref] [PubMed]


