
Tumor-induced osteomalacia secondary to phosphaturic mesenchymal tumor: a case description and literature analysis
Introduction
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome caused by the production of fibroblast growth factor 23 (FGF23) (1). The characteristic biochemical features include hypophosphatemia, significantly low levels of 1,25-dihydroxyvitamin D [1,25(OH)2D], and elevated levels of FGF23. In 70–80% of cases, TIO is caused by small, slow-growing phosphaturic mesenchymal tumors (PMTs), which may be located in almost any part of the body. TIO was first described by McCance in 1947 (2), but only just over 400 cases have been reported worldwide. PMTs are rare benign bone or soft tissue tumors with no clinical specificity and are easily missed or misdiagnosed, with an initial misdiagnosis rate of over 95% (3). Furthermore, phosphate levels are not routinely assessed in many metabolic panels, complicating early detection. Functional and anatomic imaging techniques are used to locate tumors and aid clinical diagnosis. Complete removal of these very small tumors can be curative, but locating them is often a challenge. In a case we encountered, as laboratory examinations were insufficient, we used advanced imaging techniques, including fluorine-18 aluminum fluoride-labeled octreotide positron emission tomography-computed tomography ([18F]AlF-OC PET/CT), fluorine-18 fluorodeoxyglucose PET/CT ([18F]-FDG PET/CT), computed tomography (CT), and magnetic resonance imaging (MRI), were to successfully localize the culprit tumor. TIO imposes a significant economic burden and psychological stress on both patients and their families. It is crucial to raise awareness of this condition so that more clinicians are familiar with the clinical characteristics and treatment options for TIO, as this is essential to avoiding delays in diagnosis and treatment. Early diagnosis and appropriate management can reduce complications and significantly improve the quality of life of patients with TIO.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 65-year-old male attended the hospital in 2020 with lower back and bilateral hip pain, along with weakness. Six months later, the pain in both hips worsened, causing the patient to seek treatment at the Pain Management Department of Weifang People’s Hospital. A CT scan of the lumbar spine revealed a herniated disc, and a CT scan of both hips showed osteolytic bone destruction in the left ischium (Figure 1A). The patient received minimally invasive treatment for the lumbar disc herniation, resulting in slight pain relief. One and a half years later, the patient developed chest and upper back pain, which interfered with his sleep. The symptoms progressively worsened, and 3 months later, the patient was readmitted to the pain management department. A CT scan of both hips showed a right femoral neck fracture and increased density of the left femoral neck with shortening. The left ischium’s osteolytic lesion had progressed, showing decreased density and marginal sclerosis as compared to previous imaging (March 2022) (Figure 1B). The patient was then referred to the orthopedic department, where a right total hip replacement surgery was performed (Figure 1C). Despite the surgeon’s misdiagnosis of osteoporosis, the decision was made to perform hip replacement surgery for the right femoral neck fracture in order to prevent nonunion or delayed healing of fractures caused by osteoporosis. Laboratory testing revealed a decreased serum phosphorus level (0.53 mmol/L); however, this abnormality was not recognized by the orthopedic team at the time, resulting in a delayed diagnosis of TIO. One and a half years later, the patient experienced bilateral shoulder and back pain, with radiating pain in the chest and upper back. The pain was severe enough to prevent sleep, and the patient also had persistent pain in the bilateral ribcage and sacral regions. This marked his fourth admission to the pain management department. Tramadol injections were administered for pain relief, and further functional and anatomical imaging studies were conducted. MRI revealed a well-defined, round mass in the left ischium, with low signal on T1-weighted imaging (T1WI), mixed high and low signal intensities on fat-saturated T2-weighted imaging (T2WI) (Figure 1D-1F). Whole-body bone scintigraphy imaging revealed osteoporosis and multiple fractures (Figure 2), and bone metastases needed to be excluded. [18F]-FDG PET/CT metabolic imaging revealed patchy increased radiotracer uptake in the left ischium, indicating elevated metabolic activity in this region (Figure 3A-3C). The lesion exhibited a maximum standardized uptake value (SUVmax) of 9.3, suggesting the possibility of inflammatory or malignant involvement. There were also multiple vertebral osteoporotic areas without increased radiotracer uptake (Figure 3C). Subsequently, the patient underwent [18F]AlF-OC PET/CT, which indicated intense radiotracer uptake in the lesion located in the left ischium, with an SUVmax of 4.8. These findings suggested a high expression of somatostatin receptors (SSTRs) in the lesion (Figure 3D-3F) and were used to localize the culprit tumor, confirming the diagnosis. Subsequently, the patient was readmitted to the orthopedic department for surgical intervention targeting the left ischial lesion. The preoperative laboratory data indicated the following: normal levels of 25-hydroxyvitamin D, serum calcium (Ca), parathyroid hormone (PTH), and bone metabolism index [beta-isomer of the C-terminal telopeptide cross-links of type I collagen (β-CTX) and, total procollagen type I N-terminal propeptide (T-PINP)]; decreased levels of serum potassium and phosphate; and elevated levels of alkaline phosphatase (Table 1). Due to our lack of prior experience with diagnosing TIO and the unavailability of appropriate reagents in our laboratory, caused by economic limitations, serum FGF23 was not measured. The patient received vitamin D supplementation and subsequently underwent complete surgical excision of the left ischial lesion with autologous bone grafting (Figure 1C). Upon histopathological examination, the bone marrow tissue of the ischial lesion exhibited clusters of proliferating spindle cells, along with vascular and adipose proliferation. This pattern closely resembled vascular smooth muscle lipoma, with focal cartilage-like calcifications (Figure 4A). Immunohistochemical staining showed positive cell membrane/plasma distribution of B-cell lymphoma (Bcl-2) (Figure 4B), cluster of differentiation 56 (CD56) (Figure 4C), and SSTR-2 (Figure 4D). The final diagnosis was TIO secondary to PMT. Serum phosphate and serum alkaline phosphatase levels gradually returned to normal after the operation (Table 2). After 6 months of follow-up, the tumor did not recur, and all nonspecific symptoms had disappeared.
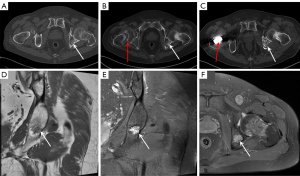
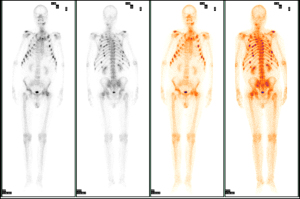
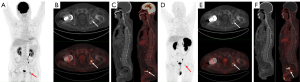
Table 1
| Item | Result | Reference range |
|---|---|---|
| ALP (U/L) | 183 | 45–125 |
| P (mmol/L) | 0.41 | 0.85–1.51 |
| Ca (mmol/L) | 2.21 | 2.11–2.58 |
| 25(OH) Vit D (ng/mL) | 27.83 | <20: deficiency; 20–30: insufficiency; >30: sufficiency |
| N-MID (ng/mL) | 11.28 | 13–48 |
| β-CTX (pg/mL) | 378.7 | 0–704 |
| T-PINP (ng/mL) | 140.8 | 15.13–58.59 |
ALP, alkaline phosphatase; Ca, calcium; N-MID, N-terminal midfragment of osteocalcin; P, phosphorus; T-PINP, total type I collagen amino-terminal propeptide; 25(OH) Vit D, 25-dihydroxy vitamin D; β-CTX, beta-isomer of the C-terminal telopeptide cross-links of type I collagen.
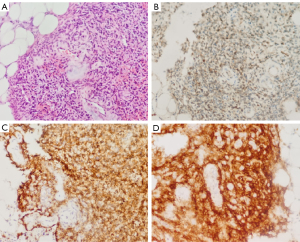
Table 2
| Item | Time | ||
|---|---|---|---|
| Preoperation | 3 days postoperation | 7 days postoperation | |
| ALP (U/L) (n: 45–125) | 183 | 166 | 161 |
| P (mmol/L) (n: 0.85–1.51) | 0.41 | 0.64 | 0.84 |
ALP, alkaline phosphatase; n, normal; P, phosphorus.
Discussion
Osteomalacia is a metabolic bone disease characterized by abnormal mineralization of newly formed bone matrix (4,5). It can be categorized into vitamin D deficiency, renal tubular acidosis, and hypophosphatemia. Low-phosphorus osteomalacia can be further divided into hereditary, neoplastic, drug-induced, and sporadic forms. TIO is a rare paraneoplastic syndrome and is secondary to PMTs in 75.5% of cases (6). PMT lesions are mostly benign, small, slow-growing, and hidden, they can occur in soft and bone tissue throughout the body. Although palpable masses are rare, most patients typically present with nonspecific, progressive symptoms, such as generalized bone pain, muscle weakness, and multiple incomplete fractures in adults or rickets and growth retardation in children. Consequently, diagnosis can be difficult, often resulting in misdiagnoses or missed diagnoses.
The primary pathogenic mechanism of TIO is the overproduction of FGF23 (7). FGF23, a phosphaturic hormone secreted by osteocytes and osteoblasts, binds to fibroblast growth factor receptors (FGFRs), primarily FGFR1, with the aid of α-Klotho, a coreceptor expressed in the kidneys, which is essential for the renal-specific action of FGF23. Excessive FGF23 leads to the internalization of sodium-phosphate cotransporters, reducing renal phosphate reabsorption and increasing phosphorus excretion through the kidneys, resulting in the failure of normal bone matrix mineralization and hypophosphatemic osteomalacia. These clinical manifestations were confirmed in the patients described in the case study. Serum FGF23 levels are useful for diagnosing TIO, monitoring tumor recurrence postsurgery, and locating the tumor source in cases of occult tumors via selective venous sampling. The primary laboratory tests for PMTs include hypophosphatemia, hyperphosphaturia, elevated levels of alkaline phosphatase, normal or decreased levels of 1,25(OH)2D, and normal levels of serum Ca and PTH. FGF23 levels decrease significantly after tumor resection. FGF23 levels are elevated in nearly all patients with TIO, although there are few reports of patients with normal FGF23 levels. Another common explanation put forth is the secretion of phosphatonins other than FGF23. Matrix extracellular phosphoglycoprotein (MEPE), secreted frizzled-related protein 4 (sFRP4), and FGF7 are the other phosphatonins that have been examined thus far. Tumors related to TIO overexpress the MEPE, sFRP4, and FGF7 genes. Their phosphaturic action has been firmly demonstrated via in vitro and animal studies, supporting their role as potential phosphatonins involved in TIO (8,9). The imaging manifestations of TIO are nonspecific, and a combination of functional and anatomical imaging is used to assist in preoperative detection and tumor localization. Functional imaging methods, including single-photon emission CT with CT (SPECT/CT) and PET/CT, are used to scan the whole body, allowing for the detection of regions with increased metabolic activity. [18F]-FDG PET/CT can be used as a sensitive tumor localization method, but it is also nonspecific (10,11). Studies have shown that SSTRs are highly expressed in most tumors responsible for TIO. The use of SSTR-targeted PET/CT imaging enables allows for the accurate identification of the culprit tumors in these patients. Recently published literature indicates that gallium 68-labeled tetraazacyclododecanetetraacetic acid-DPhe1-Tyr3-octreotat (68Ga-DOTATATE) PET/CT can be highly effective in detecting and localizing tumors in patients with TIO (8). This method exhibits a stronger affinity for the SSTR2 receptor as compared to other SSTR-based scans and demonstrates higher tumor uptake. Fluorine-18 is the most widely used PET radionuclide and offers several inherent advantages over gallium-68. Compared to 68Ga-DOTATATE, [18F]AlF-OC PET/CT provides a longer physical half-life (~110 minutes), higher spatial resolution due to a shorter positron range, and greater scalability for large-scale production and distribution (10). This imaging modality is extensively employed for tumor localization, staging, monitoring of recurrence, and assessment of treatment response (12). In our patient, the responsible tumor was also successfully located using [18F]AlF-OC PET/CT. Bone scintigraphy is also useful for the baseline assessment of patients with TIO, as it can identify fractures missed on X-ray and may aid in tumor identification. However, scintigraphy is associated with a particularly high radiation exposure, being 300 times higher than that of chest radiography (13). In the case we examined, bone scintigraphy clearly showed osteomalacia secondary to PMTs. Once functional imaging prompts suspicion of a lesion, anatomic imaging can be used to determine the location of the tumor and its surrounding tissue, thus helping the surgeon to discuss potential approaches for tumor removal. Chang et al. (14) suggested that PMTs may have certain unique imaging features. In most cases, the lesion is single, and if it is small, its density or signal is uniform. As the lesion grows, however, its density or signal becomes less uniform. Bone tumors are mostly osteolytic, and CT shows varying amounts of ground-glass opacity. In our case, CT examination revealed a fracture of the femoral neck on the right side and a hyalinous area of bone destruction on the left ischiatic bone. As the lesion progressed, sclerotic edges appeared around it, indicating a chronic noninvasive process. It did not attract sufficient attention from the radiologists, as it was mistaken for osteoporosis, which led to a misdiagnosis. The MRI findings showed heterogeneous T1WI or low-signal T2WI (almost 90% of similar cases had dark areas of T2 signal). PMTs may contain calcifications, which appear on CT as Punctate calcifications within areas of ground-glass opacity. These correspond to low signal intensity on MRI T2-weighted images and are consistent with the presence of “grungy” flocculent calcifications on histopathology. PMTs are usually significantly vascular, so most show gadolinium enhancement on MRI; however, El-Karim et al. reported a case of PMTs located in the plantar hindfoot, with no lesion enhancement on MRI and no vascular findings from histopathology (15). The CT and MRI findings in our case were consistent with those reported in the literature. Unfortunately, the patient did not undergo enhanced MRI examination. Due to its nonspecific imaging findings, TIO is easily misdiagnosed as osteoporosis, rheumatoid arthritis, delaying diagnosis and treatment.
PMTs constitute a morphologically distinctive neoplasm that is defined histologically by small, bland, spindle-to-stellate-shaped cells embedded within a myxoid or myxochondroid matrix with grungy calcification resembling chondroid or osteoid. This unique calcified matrix is the key to distinguishing PMTs from other tumors (e.g., solitary fibrous tumors, sinonasal glomangiopericytomas, nonossifying fibroma, and benign and malignant osteogenic tumors) (3,9). The patchy low signal on MRI T2WI in our patient might have been associated with pathologic grungy calcification. PMTs have prominent vascularity, with the majority of them demonstrating gadolinium enhancement on MRI (16) and showing considerable tracer uptake. Immunohistochemistry plays a limited role in recognizing PMTs due to the variable expression of several markers, including alpha-smooth muscle actin, muscle-specific actin, S-100 protein (S-100), CD34, neuron-specific enolase (NSE), CD68, synaptophysin, Bcl-2, CD56, and SSTR-2 (9). No single marker is both sensitive and specific for PMTs, making a combination of markers necessary for an accurate diagnosis. Bcl-2 helps differentiate benign from malignant lesions and aids in diagnosis when used with other markers. CD56 supports the diagnosis and is associated with a more aggressive clinical course. SSTR-2 confirms the neuroendocrine origin of PMTs and guides treatment with somatostatin analogs. Together, these markers enhance diagnostic accuracy, aid in distinguishing PMTs from other tumors, and inform clinical management decisions.
A stepwise approach is fundamental to achieving the final diagnosis (Figure 5). The classical laboratory findings in cases with TIO include hypophosphatemia resulting from renal phosphate wasting, low-to-low–normal levels of 1,25(OH)2D, and either elevated or inappropriately normal FGF-23 levels. For patients with nonspecific bone pain or evident osteomalacia, it is essential to include a fasting serum phosphate measurement (without a tourniquet) in the initial evaluation. Once hypophosphatemia is confirmed, further assessment should focus on determining renal phosphate loss. A below-normal tubular maximum phosphate reabsorption per glomerular filtration rate (TmP/GFR) or percentage of tubular reabsorption of phosphate (%TRP) indicates renal phosphate wasting. Vitamin D deficiency should be corrected prior to the calculation of TRP or TmP/GFR (17). After renal phosphate wasting has been established as the cause of a low serum phosphate level, the next crucial step in the diagnosis of TIO is the direct measurement of FGF-23 levels, for which two different assay methods are available. However, no studies have proven that one method is superior to another. Genetic testing may be necessary in certain cases that are difficult to diagnose. The case of TIO described in this report was the first we diagnosed. Due to a lack of diagnostic experience, the rarity of the disease, and factors such as economic feasibility, our laboratory lacks the relevant reagents for testing. As a result, the patient did not have serum FGF-23 tested, and neither the glomerular TmP/GFR nor the %TRP value was calculated, which led to incomplete laboratory data and a delayed diagnosis.
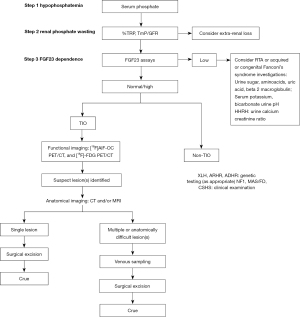
Most TIO-associated tumors are PMTs, which are considered to be benign tumors originating in the interstitial tissue and have a low nuclear grade and low mitotic activity. Complete resection of the tumor can be curative; therefore, surgery is the preferred treatment. However, several cases of recurrence with infiltration of surrounding tissue and distant metastasis to lung tissue have been reported (18,19). If the tumor is unlocalized, multifocal, unresectable, or malignant, alternative treatments are recommended. Conventional therapies include neutral phosphate and active vitamin D (e.g., alfacalcidol and calcitriol), which help alleviate symptoms but do not cure the disease. Recently, targeted therapies, such as the anti-FGF23 monoclonal antibody burosumab, have shown promise in improving symptoms and clinical indicators of TIO. In the case we encountered, no tumor recurrence or distant metastasis was found after 6 months of follow-up.
The limitations of this report include its reliance on a single case and the lack of long-term follow-up data. Several questions remain to be addressed in future research. First, the majority of PMTs display fibronectin 1-fibroblast growth factor receptor 1 (FN1-FGFR1) or fibronectin 1-fibroblast growth factor 1 (FN1-FGF1) fusions. There is a need for further investigation into the expression and function of KLOTHO, FGFR1, and the FN1-FGFR1/FGF1 fusion genes in TIO cells, as well as their role in enhancing FGF23 production. Second, the mechanisms driving FGF23 overproduction in approximately 50% of PMTs causing TIO that do not express these fusion genes require further investigation, and FN1-FGFR1/FGF1 fusion gene or KLOTHO remain to be characterized in detail.
Conclusions
TIO is most commonly caused by PMTs. Misdiagnosis, missed diagnosis, and delayed treatment are likely to occur due to its nonspecific clinical symptoms, signs, and imaging manifestations. In clinical practice, it is essential to raise awareness of the disease’s clinical characteristics, diagnostic methods, and treatment options among clinicians and optimize diagnostic and therapeutic approaches to minimize delays in diagnosis and treatment. Early diagnosis and reasonable treatment will reduce the occurrence of complications and improve the quality of life of those with TIO. A growing number of techniques are demonstrating better sensitivity and specificity for TIO, and these include 7-Tesla MRI scanners and pasireotide, which is a multireceptor-targeted somatostatin analog. However, it is necessary to conduct large-scale, high-quality clinical studies to verify their efficacy.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2278/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jan de Beur SM, Minisola S, Xia WB, Abrahamsen B, Body JJ, Brandi ML, Clifton-Bligh R, Collins M, Florenzano P, Houillier P, Imanishi Y, Imel EA, Khan AA, Zillikens MC, Fukumoto S. Global guidance for the recognition, diagnosis, and management of tumor-induced osteomalacia. J Intern Med 2023;293:309-28. [Crossref] [PubMed]
- Jiang L, Tan QQ, Gao CL, Xu L, Zhu JH, Yan PJ, Miao Y, Wan Q, Xu Y. Tumor-induced osteomalacia characterized by "painful knee joint with difficulty in moving": a case report. BMC Endocr Disord 2022;22:174. [Crossref] [PubMed]
- Minisola S, Fukumoto S, Xia W, Corsi A, Colangelo L, Scillitani A, Pepe J, Cipriani C, Thakker RV. Tumor-induced Osteomalacia: A Comprehensive Review. Endocr Rev 2023;44:323-53. [Crossref] [PubMed]
- Cianferotti L. Osteomalacia Is Not a Single Disease. Int J Mol Sci 2022;23:14896. [Crossref] [PubMed]
- Arboleya L, Braña I, Pardo E, Loredo M, Queiro R. Osteomalacia in Adults: A Practical Insight for Clinicians. J Clin Med 2023;12:2714. [Crossref] [PubMed]
- Bosman A, Palermo A, Vanderhulst J, De Beur SMJ, Fukumoto S, Minisola S, Xia W, Body JJ, Zillikens MC. Tumor-Induced Osteomalacia: A Systematic Clinical Review of 895 Cases. Calcif Tissue Int 2022;111:367-79. [Crossref] [PubMed]
- Zhang Z, Li J, Zhang Z, Shao Z. Tumor-induced Osteomalacia: A Case Report and Etiological Analysis with Literature Review. Orthop Surg 2023;15:3342-52. [Crossref] [PubMed]
- Liu S, Zhou X, Liu Y, Zhang J, Xia W. Preoperative evaluation and orthopedic surgical strategies for tumor-induced osteomalacia. J Bone Oncol 2024;45:100600. [Crossref] [PubMed]
- Folpe AL. Phosphaturic mesenchymal tumors: A review and update. Semin Diagn Pathol 2019;36:260-8. [Crossref] [PubMed]
- Liu M, Ren C, Zhang H, Zhang Y, Huang Z, Jia R, Cheng Y, Bai C, Xu Q, Zhu W, Huo L. Evaluation of the safety, biodistribution, dosimetry of [18F]AlF-NOTA-LM3 and head-to-head comparison with [68Ga]Ga-DOTATATE in patients with well-differentiated neuroendocrine tumors: an interim analysis of a prospective trial. Eur J Nucl Med Mol Imaging 2024;51:3719-30. [Crossref] [PubMed]
- Abadi Y, Mileva M, Léger MA, Sidiras P, Artigas C, Flamen P, Karfis I. Phosphaturic mesenchymal tumor demonstrated by (68)Ga-DOTATATE PET/CT in a patient: a case report. EJNMMI Rep 2024;8:30. [Crossref] [PubMed]
- Boeckxstaens L, Pauwels E, Vandecaveye V, Deckers W, Cleeren F, Dekervel J, Vandamme T, Serdons K, Koole M, Bormans G, Laenen A, Clement PM, Geboes K, Van Cutsem E, Nackaerts K, Stroobants S, Verslype C, Van Laere K, Deroose CM. Prospective comparison of [18F]AlF-NOTA-octreotide PET/MRI to [68Ga]Ga-DOTATATE PET/CT in neuroendocrine tumor patients. EJNMMI Res 2023;13:53. [Crossref] [PubMed]
- Dahir K, Zanchetta MB, Stanciu I, Robinson C, Lee JY, Dhaliwal R, Charles J, Civitelli R, Roberts MS, Krolczyk S, Weber T. Diagnosis and Management of Tumor-induced Osteomalacia: Perspectives From Clinical Experience. J Endocr Soc 2021;5:bvab099. [Crossref] [PubMed]
- Chang C, Yu A, You Y, Peng X, Cheng X, Li X, Liang W, Gong L, Deng W. Clinical and imaging features of phosphaturic mesenchymal tumors. Chin Med J (Engl) 2023;136:351-3. [Crossref] [PubMed]
- El-Karim GA, Almalki Y, Alolabi B. Stumbling upon the unexpected: A unique presentation of phosphaturic mesenchymal tumor in the hindfoot. Radiol Case Rep 2020;15:858-62. [Crossref] [PubMed]
- Brandi ML, Clunie GPR, Houillier P, Jan de Beur SM, Minisola S, Oheim R, Seefried L. Challenges in the management of tumor-induced osteomalacia (TIO). Bone 2021;152:116064. [Crossref] [PubMed]
- Jadhav SS, Shah R, Patil V. Tumor-induced osteomalacia: An overview. Best Pract Res Clin Endocrinol Metab 2024;38:101834. [Crossref] [PubMed]
- Yan J, Jiang J, Wu X, Zhou L. Progressive bone pain caused by a phosphaturic mesenchymal tumor in the left femur: a case report and literature review. J Int Med Res 2024;52:3000605241285540. [Crossref] [PubMed]
- Abate V, Vergatti A, De Filippo G, Damiano V, Menale C, D'Elia L, Rendina D. Clinical Characteristics of Malignant Phosphaturic Mesenchymal Tumor Causing Tumor-Induced Osteomalacia. J Clin Endocrinol Metab 2024;109:e1006-11. [Crossref] [PubMed]


