
Prognostic value of angiography-derived fractional flow reserve and translesion gradient after drug-coated balloon angioplasty
Introduction
Drug-coated balloons (DCBs) have emerged as a beneficial treatment for coronary artery disease, particularly in the context of in-stent restenosis (ISR) and small vessel disease, providing an alternative to drug-eluting stents by delivering antiproliferative drugs directly to the vessel wall without the requirement of a permanent implant (1-5). However, the efficacy of DCB therapy can vary, and there is a need for immediate postprocedural assessment tools that can predict long-term outcomes.
Fractional flow reserve (FFR) is the gold standard for guiding interventional treatments (6). Angiography-based FFR (angio-FFR) estimations, such as angiography-derived fractional flow reserve (AccuFFRangio) (7), quantitative flow ratio (QFR) (8,9), and FFRangio (10), are recent advancements that use standard angiographic images to calculate FFR without additional invasive instrumentation (11). AccuFFRangio has been shown to have clinical efficacy, demonstrating good correlation and agreement with traditional wire-based FFR measurements (12,13).
Physiological assessments after percutaneous coronary intervention (PCI) are being increasingly performed both in the context of clinical studies and routine practice (14). Post-PCI FFR has been proposed as a metric that can reflect the degree of residual flow limitation in coronary vessels. Several studies have demonstrated that post-PCI FFR values correlate with risk of major adverse cardiac events, underscoring the importance of achieving optimal physiological results during the initial procedure (15-17). Conversely, the significance of angio-FFR immediately after DCB treatment for assessing coronary flow has not been extensively investigated, and data on this specific angio-FFR application are sparse. The assessment of translesion pressure gradients is being increasingly recognized for its potential to predict long-term outcomes (18-20). However, the requirement for a motorized pressure wire pullback device during continuous hyperemia may hinder its clinical adoption. AccuFFRangio technology can generate a virtual pullback curve, illustrating the pressure at each point along the interrogated vessel. A recent study reported that the integration of pre-PCI and post-PCI physiological information could provide improved risk stratification compared to clinical factors alone in vessels with stent implantation (21).
The prognostic value of immediate postprocedural AccuFFRangio following DCB application has not been thoroughly investigated. Moreover, the translesion gradient (TLG), measured as the difference in AccuFFRangio across a lesion, may provide additional predictive insights into long-term outcomes such as target vessel failure (TVF). To provide a novel perspective on postintervention assessment, this study aimed to determine the value of immediate post-DCB AccuFFRangio values and TLG in predicting 2-year TVF. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2238/rc).
Methods
Study design and population
This retrospective, single-center analysis included patients who underwent PCI with DCB at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University between January 2020 and January 2022. Patients with hemodynamic instability, cardiogenic shock, urgent revascularization, lesions involving the left main coronary artery, or coronary bypass grafting were excluded. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by institutional ethics committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (No. 2022-K-39-01) and informed consent was obtained from all individual participants.
AccuFFRangio and TLG measurement
AccuFFRangio and TLG were calculated using dedicated software (AccuFFRangio V1.0, ArteryFlow Technology, Hangzhou, China) by well-trained technicians in a blinded manner (22). Coronary angiographic images for AccuFFRangio computation were acquired immediately after DCB angioplasty to assess the acute hemodynamic effects of the intervention. However, the actual computation was performed offline by an independent core laboratory after the procedure was completed, ensuring standardized analysis while avoiding intraprocedural delays. Briefly, the three-dimensional (3D) anatomical model of the vessel of interest was reconstructed automatically using two angiographic views with projections ≥25° apart. The contrast frame count was performed to obtain the blood flow velocity as an inlet boundary condition. Computational fluid dynamics were then applied to compute the pressure drop across the segmented vessel, enabling AccuFFRangio reading at any point along the vessel. TLG was defined as the difference in AccuFFRangio between the proximal and distal segments of the interrogated lesion where DCB treatment was performed. Each target vessel was analyzed independently by two technicians. If the difference between their AccuFFRangio values exceeded 0.03, the results were reviewed, discussed, and recalculated until a consensus was reached. The final AccuFFRangio value for each vessel was determined as the mean of the two independent measurements. The interrater agreement between operators was very high in all cases (κ>0.95), demonstrating excellent reproducibility.
Quantitative coronary analysis (QCA)
QCA was performed by two experienced radiologists without access to the AccuFFRangio results. Post-DCB minimum lumen diameter (MLD), percentage diameter stenosis (%DS), and the dissection classifications were assessed for all vessels. All discrepancies were resolved through discussion until a consensus was reached.
Clinical follow-up
Clinical follow-up was performed through outpatient services or telephone contact at 1, 6, 12, and 24 months. The primary endpoint was TVF, defined as a composite of cardiac death, target vessel myocardial infarction (MI), and clinically driven target vessel revascularization at 2 years after the indexed procedures. Procedural and clinical data on all patients were collected by local investigators and recorded on electronic case-report forms. A central monitoring team verified all submitted information.
Statistical analysis
Categorical variables are reported as numbers and percentages and were compared with the chi-squared test. The D’Agostino Pearson test was used to test the normality of the data. All continuous variables are presented as the mean and standard deviation (SD) or as the median and interquartile range (IQR), according to their distribution. For continuous variables, the Mann-Whitney test was used for independent samples, the Wilcoxon test for paired samples, and the Friedman test for more than two paired samples. The predictive value of post-DCB AccuFFRangio and TLG for 2-year TVF was analyzed via receiver operating characteristic (ROC) curves, with the cutoff values of AccuFFRangio and TLG, sensitivity, specificity, area under the curve (AUC), 95% confidence interval (CI), and P value being reported. Patients were then classified according to AccuFFRangio and TLG cutoff value. Event-free survival rates were estimated via Kaplan-Meier analysis and compared with the log-rank test. Predictors of TVF were analyzed using the Cox proportional hazards model. Statistical significance was defined as a two-sided P value <0.05. All analyses were performed with SPSS version 26.0.0 (IBM Corp., Armonk, NY, USA).
Results
Patient baseline characteristics and clinical outcome
Our study included 232 patients undergoing DCB angioplasty. Post-DCB AccuFFRangio measurements were unavailable in 6 patients due to technical limitations: 4 cases had excessive vessel overlap or foreshortening, preventing accurate delineation of proximal/distal references, and 2 cases lacked dual angiographic projections with ≥25° separation, which are required for 3D reconstruction. At the 2-year follow-up after the index procedure, 8 patients were lost, leaving 218 (93.9%) patients with complete post-DCB AccuFFRangio and clinical follow-up data (Figure 1). The mean age was 69±10 years, with males constituting 71% of the cohort. The prevalence of diabetes mellitus and hypertension was 40% and 68%, respectively. Among the lesions assessed, 66.5% were de novo, while 33.5% were ISR lesions, predominantly located in the left anterior descending artery (LAD) (Table 1). At 2 years after DCB treatment, TVF occurred in 19 (8.7%) patients, resulting in 1 case of cardiac death, 2 cases of target vessel-related MI, and 16 cases of target vessel revascularization.
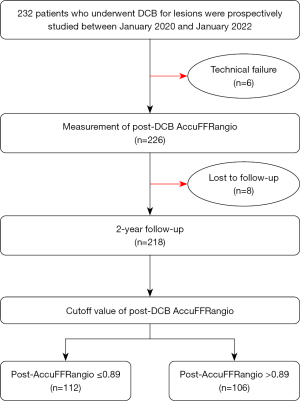
Table 1
| Variable | Overall | AccuFFRangio ≤0.89 (n=112) |
AccuFFRangio >0.89 (n=106) |
P value |
|---|---|---|---|---|
| Characteristic | ||||
| Age, years | 68.9±10.2 | 69.0±10.2 | 68.8±10.3 | 0.862 |
| Female | 62 [28] | 37 [33] | 25 [24] | 0.051 |
| Diabetes mellitus | 85 [39] | 39 [35] | 46 [43] | 0.491 |
| Hypertension | 148 [68] | 74 [66] | 74 [70] | 0.663 |
| Hyperlipidemia | 56 [26] | 31 [28] | 25 [24] | 0.351 |
| Smoking | 92 [42] | 44 [39] | 48 [45] | 0.891 |
| BMI, kg/m2 | 23.7±5.9 | 24.8±4.1 | 22.6±7.0 | 0.007 |
| SBP, mmHg | 136.2±24.5 | 135.2±27.7 | 137.3±20.8 | 0.536 |
| DBP, mmHg | 77.1±11.6 | 76.5±11.6 | 77.6±11.7 | 0.489 |
| LDL-c, mmol/L | 2.4±1.1 | 2.4±1.0 | 2.4±1.2 | 0.924 |
| HDL-c, mmol/L | 1.0±0.3 | 1.0±0.3 | 1.0±0.3 | 0.567 |
| SCr, mmol/L | 77.4±25.1 | 76.0±22.5 | 78.8±27.5 | 0.428 |
| LVEF, % | 62.1±10.3 | 61.2±11.4 | 63.0±9.0 | 0.226 |
| Lesion characteristics | ||||
| Lesion type | 0.316 | |||
| De novo | 145 [67] | 71 [63] | 74 [70] | |
| ISR | 73 [33] | 41 [37] | 32 [30] | |
| Lesion location | 0.272 | |||
| LAD | 71 [33] | 40 [36] | 31 [29] | |
| D | 25 [11] | 8 [7] | 17 [16] | |
| LCX | 53 [24] | 30 [27] | 23 [22] | |
| OM | 11 [5] | 4 [3] | 7 [7] | |
| RCA | 48 [22] | 24 [21] | 24 [23] | |
| PDA/PLA | 10 [5] | 6 [5] | 4 [4] | |
Values are expressed as the mean ± SD or n [%]. AccuFFRangio, angiography-derived fractional flow reserve; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; ISR, in-stent restenosis; LVEF, left ventricular ejection fraction; SCr, serum creatine; LAD, left anterior descending artery; D, diagonal branches; LCX, left circumflex artery; OM, obtuse marginal branch; RCA, right coronary artery; PDA, posterior descending artery; PLA, posterolateral artery; DCB, drug-coated balloon; SD, standard deviation.
Post-DCB AccuFFRangio analysis
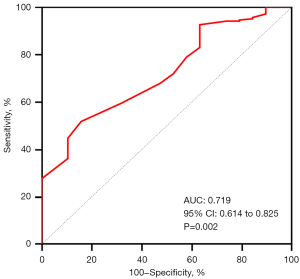
The median value of post-DCB AccuFFRangio was 0.89 (IQR, 0.86–0.93). The ROC curve analysis identified an optimal cutoff value of post-DCB AccuFFRangio of 0.89 for predicting TVF, with a sensitivity of 85% and a specificity of 52% (AUC =0.719; P=0.002) (Figure 2). Based on this cutoff, patients were stratified into two groups: those with post-AccuFFRangio ≤0.89 (n=112, 51%) and those with post-AccuFFRangio >0.89 (n=106, 49%).
There were no significant differences in baseline demographics or clinical risk factors between the groups (Table 1). Angiographic and procedural characteristics, including DCB dimensions and deployment parameters, were also comparable. However, the post-AccuFFRangio ≤0.89, as compared to the post-AccuFFRangio >0.89 group, exhibited a smaller MLD (1.6±0.5 vs. 2.1±0.5 mm; P<0.001) and a higher DS (34.3%±10.4% vs. 24.1%±8.5%; P<0.001) (Table 2).
Table 2
| Variable | Overall | AccuFFRangio ≤0.89 (n=112) |
AccuFFRangio >0.89 (n=106) |
P value |
|---|---|---|---|---|
| Procedural | ||||
| DCB diameter, mm | 2.6±0.7 | 2.6±0.5 | 2.6±0.9 | 0.899 |
| DCB length, mm | 24.0±6.2 | 25.0±5.9 | 23.0±6.3 | 0.016 |
| DCB pressure, atm | 8.5±1.8 | 8.3±1.4 | 8.6±2.1 | 0.213 |
| DCB time, s | 55.6±14.7 | 53.5±17.1 | 57.5±12.3 | 0.161 |
| Dissection after balloon treatment | 0.665 | |||
| Type A | 90 (41.3) | 48 (42.8) | 42 (39.6) | |
| Type B | 44 (20.2) | 21 (18.8) | 23 (21.7) | |
| Type C | 4 (1.8) | 2 (1.8) | 2 (1.9) | |
| AccuFFRangio and QCA measurement | ||||
| Post-DCB AccuFFRangio | 0.88±0.06 | 0.84±0.06 | 0.93±0.02 | <0.001 |
| Post-DCB TLG | 0.04±0.04 | 0.06±0.04 | 0.03±0.03 | <0.001 |
| Post-DCB MLD, mm | 1.8±0.5 | 1.6±0.5 | 2.1±0.5 | <0.001 |
| Post-DCB DS, % | 29.3±10.8 | 34.3±10.4 | 24.1±8.5 | <0.001 |
Values are expressed as n (%) or mean ± SD. AccuFFRangio, angiography-derived fractional flow reserve; DCB, drug-coated balloon; QCA, quantitative coronary angiography; MLD, minimum lumen diameter; DS%, percent diameter stenosis; TLG, translesion gradient; SD, standard deviation.
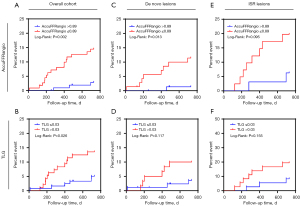
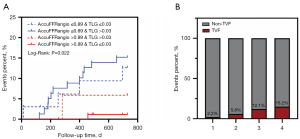
Kaplan-Meier analysis indicated that a lower post-DCB AccuFFRangio (≤0.89) was associated with a significantly higher rate of TVF (14.3% vs. 2.8%; P=0.002) (Figure 3A). Furthermore, when vessels were stratified according to the median value of TLG (>0.03), we found that vessels with a high TLG index had a higher risk of TVF than did those with a low TLG index (13.5% vs. 4.9%; P=0.026) (Figure 3B). Further subgroup analysis by lesion type revealed the following: Among those with de novo lesions (n=145), patients with AccuFFRangio ≤0.89 (n=71) exhibited significantly higher TVF rates than did those with AccuFFRangio >0.89 (11.3% vs. 1.4%; P=0.013; Figure 3C) although stratification by TLG (threshold ≤0.03) indicated no significant difference in TVF (11.1% vs. 3.5%; P=0.117; Figure 3D). In ISR lesions (n=73), a tendency for higher TVF risk was observed in the AccuFFRangio ≤0.89 subgroup as compared to the >0.89 subgroup (19.5% vs. 6.3%; P=0.095; Figure 3E), while stratification by TLG (≤0.03) indicated a numerically higher TVF rate in the low TLG group than in the high TLG group (19.4% vs. 8.1%; P=0.155; Figure 3F) although there was no statistical significance in either subgroup analysis, likely due to the limited sample size.
Factors associated with TVF
Univariate Cox regression analysis revealed that the factors associated with subsequent TVF were male gender [hazard ratio (HR): 7.501, 95% CI: 1.001–56.191; P=0.050], smoking (HR: 2.887, 95% CI: 1.083–7.694; P=0.034), post-DCB AccuFFRangio (HR: 0.415, 95% CI: 0.271–0.635; P<0.001), post-DCB TLG (HR: 1.103, 95% CI: 1.030–1.181; P=0.005), and residual DS (HR: 1.052, 95% CI: 1.014–1.090; P=0.007) (Table 3). In the multivariate analysis adjusted for significant variables, post-DCB AccuFFRangio remained an independent predictor of TVF. However, when post-DCB TLG was added to the baseline clinical model, post-DCB AccuFFRangio ceased to be an independent predictor (Table 4).
Table 3
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age, years | 0.993 | 0.949–1.038 | 0.743 |
| Male sex | 7.501 | 1.001–56.191 | 0.050 |
| Diabetes mellitus | 0.637 | 0.253–1.606 | 0.339 |
| Hypertension | 1.370 | 0.531–3.535 | 0.515 |
| Hyperlipidemia | 1.761 | 0.510–6.083 | 0.371 |
| Smoking | 2.887 | 1.083–7.694 | 0.034 |
| BMI, kg/m2 | 0.995 | 0.923–1.072 | 0.890 |
| SBP, mmHg | 0.999 | 0.980–1.019 | 0.941 |
| DBP, mmHg | 0.985 | 0.947–1.024 | 0.433 |
| LDL-c, mmol/L | 0.825 | 0.494–1.378 | 0.461 |
| HDL-c, mmol/L | 0.289 | 0.043–1.987 | 0.207 |
| SCr, mmol/L | 1.000 | 0.981–1.019 | 0.992 |
| LVEF, % | 1.005 | 0.952–1.060 | 0.860 |
| Procedural | |||
| DCB diameter, mm | 1.567 | 0.555–4.424 | 0.396 |
| DCB length, mm | 1.013 | 0.942–1.089 | 0.726 |
| DCB pressure, atm | 1.018 | 0.785–1.320 | 0.893 |
| DCB time, s | 1.035 | 0.980–1.092 | 0.227 |
| Dissection after balloon treatment | |||
| Type A | 0.428 | 0.129–1.421 | 0.166 |
| Type B | 1.580 | 0.573–4.358 | 0.337 |
| Type C | – | – | – |
| AccuFFRangio and QCA measurement | |||
| Post-DCB AccuFFRangio, per 0.01 | 0.415 | 0.271–0.635 | <0.001 |
| Post-DCB TLG, per 0.01 | 1.103 | 1.030–1.181 | 0.005 |
| Post-DCB MLD, per 0.1 mm | 0.924 | 0.841–1.015 | 0.101 |
| Residual %DS, per 1% | 1.052 | 1.014–1.090 | 0.007 |
AccuFFRangio, angiography-derived fractional flow reserve; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DCB, drug-coated balloon; DS%, percent diameter stenosis; HDL-c, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-c, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MLD, minimum lumen diameter; QCA, quantitative coronary angiography; SCr, serum creatine; SBP, systolic blood pressure; SD, standard deviation; TLG, translesion gradient.
Table 4
| Variable | Post-PCI AccuFFRangio model | AccuFFRangio and TLG model | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Male sex | 4.019 | 0.465–34.770 | 0.206 | 4.108 | 0.476–35.440 | 0.199 | |
| Smoking yes | 1.825 | 0.642–5.189 | 0.259 | 1.776 | 0.625–5.043 | 0.625 | |
| Post-DCB DS | 1.007 | 0.957–1.060 | 0.779 | 1.002 | 0.951–1.056 | 0.935 | |
| Post-DCB AccuFFRangio, per 0.01 | 0.926 | 0.863–0.994 | 0.033 | 0.936 | 0.867–1.012 | 0.096 | |
| Post-DCB TLG, per 0.01 | 1.048 | 0.918–1.196 | 0.488 | ||||
AccuFFRangio, angiography-derived fractional flow reserve; CI, confidence interval; DCB, drug-coated balloon; DS, diameter stenosis; HR, hazard ratio; PCI, percutaneous coronary intervention; TLG, translesion gradient.
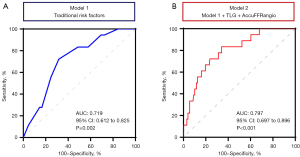
Notably, when post-DCB AccuFFRangio and TLG were added as continuous variables to a model with conventional risk factors (including gender, smoking, diabetes, and hypertension, model 1), the expanded model 2 showed a significantly higher discrimination ability for predicting the occurrence of TVF (P<0.001) (Figure 4). However, incorporating %DS into model 2 did not further improve the predictive performance, reinforcing the superiority of functional parameters over anatomical stenosis assessment in risk stratification (ΔAUC: −0.01; P=0.40).
Subgroup analysis
Patients were grouped according to the cutoff value of post-DCB AccuFFRangio (≤0.89) and the median of the TLG (>0.03) into four categories: group 1, AccuFFRangio >0.89 and TLG ≤0.03; group 2, AccuFFRangio >0.89 and TLG >0.03; group 3, AccuFFRangio ≤0.89 and TLG ≤0.03; and group 4, AccuFFRangio ≤0.89 and TLG >0.03. There were 89 patients in group 1, 17 in group 2, 33 in group 3, and 79 in group 4. The detailed patient characteristics of these groups are presented in Table S1. Baseline characteristics were well balanced among the 4 groups, with the exception of some important angiographic differences. The subgroup with high TLG and low AccuFFRangio had a significantly higher rate of TVF (15.2%) as compared to patients with (I) low TLG and low AccuFFRangio (TVF 12.1%); (II) high TLG and high AccuFFRangio (TVF 5.8%), and (III) low TLG and high AccuFFRangio (TVF 2.2%) (Figure 5). Additionally, after stratification by post-DCB AccuFFRangio and angiographically defined dissection type B or beyond, subsequent TVF rates were markedly higher in lesions with both post-DCB AccuFFRangio ≤0.89 and dissection type B or beyond as compared to those without post-DCB AccuFFRangio ≤0.89 or significant dissection (P<0.001) (Figure S1).
Discussion
This study aimed to determine the cutoff value of post-DCB AccuFFRangio for the predicting long-term clinical events and to investigate the association of long-term adverse events with AccuFFRangio alone and in combination with AccuFFRangio-based TLG. The principal findings of our study were as follows: (I) the cutoff value of post-DCB AccuFFRangio for predicting TVF was 0.89, and a post-DCB AccuFFRangio ≤0.89 was strongly associated with TVF at 2 years postprocedure in the overall patient population; (II) post-DCB AccuFFRangio was an independent predictor of TVF; and (III) the combination of low AccuFFRangio and high TLG predicted the highest TVF rate.
Recent studies have demonstrated that DCB is noninferior to drug-eluting stents in treating native coronary arteries in specific populations, particularly in treating ISR and small vessel disease (1-5). Although DCB offers a promising alternative to traditional stent implantation for treating coronary artery disease, it has several limitations. Severe dissections often necessitate subsequent stenting, and DCB is less effective for complex lesions such as diffuse, calcified, and tortuous ones, which can lead to acute vessel closure (23). Given that numerous factors influence the therapeutic efficacy of DCB, immediate hemodynamic assessment after DCB treatment has recently garnered significant interest among clinicians.
In routine clinical practice, the assessment of PCI outcomes relies predominantly on angiographic appearance, but this can often overlook residual flow impairment factors such as diffuse or localized narrowing, especially at the proximal or distal ends (24,25). Invasive coronary artery imaging is critical to characterizing the pathophysiological mechanisms underlying impaired coronary blood flow and in guiding appropriate interventions (26). Diletti et al. reported that a post-PCI FFR <0.90 was correlated with higher rates of TVF and could be linked to an increased rate of stent thrombosis over a 2-year follow-up (16). However, FFR’s widespread adoption in clinical practice remains limited primarily due to the absence of well-defined thresholds and the requirement for prolonged adenosine infusion, which may cause patient discomfort and unstable hyperemia, complicating the identification of focal or diffuse lesions (19,27-29). Recently, several studies have demonstrated that imaging-based post-PCI FFR has comparable effectiveness and accuracy to conventional FFR in predicting postprocedure adverse cardiovascular events (14,21,30,31). In our study, ROC analysis identified a cutoff value of 0.89 for AccuFFRangio in predicting TVF events. We stratified patients based on immediate post-DCB AccuFFRangio values and found that those with values ≤0.89 had a significantly higher risk of TVF. Subgroup analysis revealed that both TLG and AccuFFRangio exhibited similar prognostic tendencies across lesion types; however, statistical significance was not reached for AccuFFRangio in ISR lesions (P=0.095) or for TLG in de novo (P=0.117) and ISR lesions (P=0.155), which is likely attributable to insufficient sample size. This underscores the need for larger cohorts to validate the predictive utility of these parameters in specific lesion subsets. These findings not only align with previous research (13) demonstrating the importance of AccuFFRangio in guiding PCI but also highlight the specific utility of Angio-FFR in the context of DCB interventions, suggesting that even in the absence of stent placement, achieving optimal Angio-FFR values is crucial for preventing long-term complications. A recent multicenter study by Jiang et al. further validated the high diagnostic accuracy of AccuFFRangio in identifying hemodynamically significant lesions (95.07%), supporting it as a reliable tool for functional assessment in a diversity of clinical settings (13). Unlike stent implantation, DCB treatment requires the consideration of acute recoil and dissection, necessitating evaluation of postprocedural timing. Immediate measurements enable real-time feedback for intraprocedural decisions, aligning with clinical workflows, while 15 minute-delayed measurements allow for vasomotor recovery and potentially a more accurate reflection of the physiology (14). However, delayed assessments prolong procedural time, limiting practicality. Future studies should systematically compare immediate and delayed measurements to optimize the timing of physiological assessment.
TLG is a measure of the pressure difference across a lesion and has been studied as an indicator of the severity and physiological impact of coronary artery stenosis (19,20). An additional benefit of Angio-FFR is its automatic provision of a comprehensive vessel pullback, offering detailed point-by-point insights into the functional significance of specific stenoses, enabling a more robust comparison of TLG than traditional FFR. Previous studies have shown that preoperative translesion pressure gradients can assess the physiological patterns of vessels (19,21). However, in our study, we evaluated TLG immediately after DCB treatment, and using this index to assess the physiological patterns of vessels seems inappropriate. A larger TLG indicates a greater net force on the plaque. Since axial plaque stress is influenced by both pressure and lesion geometry, plaques with the same TLG may undergo different stress levels based on variations in lesion geometry (32). In our study, we found that higher TLG values post-DCB are associated with increased risks of restenosis and adverse cardiac events, which is in line with a previous study on stent implantation (18). Moreover, in subgroup analysis, we found that patients with a high TLG and low AccuFFRangio had significantly higher rates of TVF (15.2%). Furthermore, despite achieving favorable physiological outcomes following DCB treatment (AccuFFRangio >0.89), patients with local TLG >0.03 after DCB were associated with worse clinical outcomes as compared to those with low TLG, although the difference was not statistically significant.
In summary, as the field of interventional cardiology continues to refine its strategies and improve patient outcomes, the role of advanced physiological assessments such as AccuFFRangio and TLG is becoming increasingly pivotal. A comprehensive and carefully conducted AccuFFRangio assessment post-DCB treatment can likely provide diagnostic and prognostic value. This approach could potentially assist clinicians in enhancing the degree of functional revascularization, thereby improving patient management and long-term outcomes.
Limitations
Although our study provides valuable insights, it is not without certain limitations. First, the single-center design may reduce the generalizability of the findings, notably the low specificity of the AccuFFRangio cutoff value. Future multicenter studies with larger sample sizes are necessary to validate our results. Second, several technical factors can influence the accuracy of AccuFFRangio measurements, such as image quality and operator experience. These factors need careful management, and their influence warrants further investigation, especially regarding diagnostic performance in cases of angiographically significant dissection. Third, the optimal cutoff value for post-DCB AccuFFRangio was determined solely based on our study population. This cutoff value should be validated in larger, more diverse populations to confirm its utility and accuracy in clinical practice. Fourth, the study did not address the diagnostic accuracy of AccuFFRangio in complex lesion subsets such as tandem lesions, bifurcation lesions, and long lesions. These types of lesions could potentially affect the cutoff value of AccuFFRangio, suggesting a need for further studies focusing on these complex cases. In addition, in this study, no postdilation was performed in cases with low AccuFFRangio values (≤0.89), as interventions were deferred to real-world clinical judgment. This design limitation precludes definitive conclusions regarding the potential benefits of adjunctive postdilation in improving outcomes for these patients. Finally, further research is needed to determine how the timing of AccuFFRangio measurements affects the prediction of long-term outcomes. Determining whether immediate postprocedure measurements and delayed measurements (e.g., 15 minutes after PCI) differ in their predictive value could refine the use of AccuFFRangio in clinical practice. Overall, our findings provide valuable insights into the use of AccuFFRangio following DCB angioplasty, but they need to be validated and expanded upon in prospective, multicenter studies with predefined clinical endpoints to enhance their applicability and reliability in broader clinical settings.
Conclusions
Our findings indicate that post-DCB AccuFFRangio and TLG are valuable for predicting the long-term clinical outcomes in patients undergoing coronary DCB angioplasty. The independent predictive value of AccuFFRangio, especially when used in conjunction with traditional risk factors, underscores its potential utility in enhancing postprocedural patient stratification and management. These results suggest that AccuFFRangio and TLG may offer valuable insights beyond conventional angiographic assessment. Future studies should explore the integration of these metrics into routine clinical practice and investigate their impact on decision-making processes to optimize patient outcomes following DCB angioplasty.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2238/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2238/coif). Y.H. and J.X. report that they are employees of ArteryFlow Technology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by institutional ethics committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (No. 2022-K-39-01) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang W, Zhang M, Tian J, Zhang M, Zhou Y, Song X. Drug-Coated Balloon-Only Strategy for De Novo Coronary Artery Disease: A Meta-analysis of Randomized Clinical Trials. Cardiovasc Ther 2023;2023:3121601. [Crossref] [PubMed]
- Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J, Scheller B, Kleber FXInternational DCB Consensus Group. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv 2020;13:1391-402. [Crossref] [PubMed]
- Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet 2018;392:849-56. [Crossref] [PubMed]
- Sinaga DA, Ho HH, Watson TJ, Sim A, Nyein TT, Jafary FH, Loh JK, Ooi YW, Tan JK, Ong PJ. Drug-Coated Balloons: A Safe and Effective Alternative to Drug-Eluting Stents in Small Vessel Coronary Artery Disease. J Interv Cardiol 2016;29:454-60. [Crossref] [PubMed]
- Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Weilenmann D, Wöhrle J, Stachel G, Markovic S, Leibundgut G, Rickenbacher P, Osswald S, Cattaneo M, Gilgen N, Kaiser C, Scheller B. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020;396:1504-10. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. [Crossref] [PubMed]
- Jiang J, Tang L, Du C, Leng X, He J, Hu Y, Dong L, Sun Y, Li C, Xiang J, Wang J. Diagnostic performance of AccuFFRangio in the functional assessment of coronary stenosis compared with pressure wire-derived fractional flow reserve. Quant Imaging Med Surg 2022;12:949-58. [Crossref] [PubMed]
- Tanigaki T, Emori H, Kawase Y, Kubo T, Omori H, Shiono Y, Sobue Y, Shimamura K, Hirata T, Matsuo Y, Ota H, Kitabata H, Okubo M, Ino Y, Matsuo H, Akasaka T. QFR Versus FFR Derived From Computed Tomography for Functional Assessment of Coronary Artery Stenosis. JACC Cardiovasc Interv 2019;12:2050-9. [Crossref] [PubMed]
- Biscaglia S, Verardi FM, Tebaldi M, Guiducci V, Caglioni S, Campana R, et al. QFR-Based Virtual PCI or Conventional Angiography to Guide PCI: The AQVA Trial. JACC Cardiovasc Interv 2023;16:783-94. [Crossref] [PubMed]
- Fearon WF, Achenbach S, Engstrom T, Assali A, Shlofmitz R, Jeremias A, Fournier S, Kirtane AJ, Kornowski R, Greenberg G, Jubeh R, Kolansky DM, McAndrew T, Dressler O, Maehara A, Matsumura M, Leon MB, De Bruyne B. Accuracy of Fractional Flow Reserve Derived From Coronary Angiography. Circulation 2019;139:477-84. [Crossref] [PubMed]
- Berry C, Ang DTY. Picture perfect? Performance of quantitative coronary angiography-based vessel FFR versus pressure wire-based FFR. EuroIntervention 2022;17:1463-5. [Crossref] [PubMed]
- Li C, Hu Y, Wang J, Pan C, Lu H, Wu Y, Chen Z, Pei Z, Shen L, He J, Leng X, Xiang J, Ge J. Are baseline conditions of coronary arteries sufficient for calculating angio-based index of microcirculatory resistance and fractional flow reserve? Quant Imaging Med Surg 2023;13:6215-27. [Crossref] [PubMed]
- Jiang J, Hu Y, Li C, Dong L, Xu J, Tang L, Jiang W, Du C, Jiang X, Lyu Y, Leng X, Li C, Koo BK, Xiang J, Ge J, Wang J. Diagnostic Accuracy of Computational Fluid Dynamics-Based Fractional Flow Reserve Derived From Coronary Angiography: The ACCURATE Study. J Am Heart Assoc 2025;14:e035672. [Crossref] [PubMed]
- Yamamoto T, Ishii T, Ishida A. Impact of post physiological assessment after treatment for de novo coronary lesions using drug-coated balloons. Int J Cardiol 2022;363:11-9. [Crossref] [PubMed]
- Collet C, Johnson NP, Mizukami T, Fearon WF, Berry C, Sonck J, et al. Impact of Post-PCI FFR Stratified by Coronary Artery. JACC Cardiovasc Interv 2023;16:2396-408. [Crossref] [PubMed]
- Diletti R, Masdjedi K, Daemen J, van Zandvoort LJC, Neleman T, Wilschut J, Den Dekker WK, van Bommel RJ, Lemmert M, Kardys I, Cummins P, de Jaegere P, Zijlstra F, Van Mieghem NM. Impact of Poststenting Fractional Flow Reserve on Long-Term Clinical Outcomes: The FFR-SEARCH Study. Circ Cardiovasc Interv 2021;14:e009681. [Crossref] [PubMed]
- Collison D, Didagelos M, Aetesam-Ur-Rahman M, Copt S, McDade R, McCartney P, et al. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGET-FFR). Eur Heart J 2021;42:4656-68. [Crossref] [PubMed]
- Dai N, Hwang D, Lee JM, Zhang J, Jeon KH, Paeng JC, Cheon GJ, Koo BK, Ge J. Feasibility of Quantitative Flow Ratio-Derived Pullback Pressure Gradient Index and Its Impact on Diagnostic Performance. JACC Cardiovasc Interv 2021;14:353-5. [Crossref] [PubMed]
- Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, Argacha JF, Schoors D, Colaiori I, Di Gioia G, Kodeboina M, Suzuki H, Van 't Veer M, Bartunek J, Barbato E, Cosyns B, De Bruyne B. Measurement of Hyperemic Pullback Pressure Gradients to Characterize Patterns of Coronary Atherosclerosis. J Am Coll Cardiol 2019;74:1772-84. [Crossref] [PubMed]
- Uretsky BF, Agarwal SK, Vallurupalli S, Al-Hawwas M, Miller K, Biscaglia S, Hakeem A. Trans-Stent FFR Gradient as a Modifiable Integrant in Predicting Long-Term Target Vessel Failure. JACC Cardiovasc Interv 2022;15:2192-202. [Crossref] [PubMed]
- Dai N, Yuan S, Dou K, Zhang R, Hu N, He J, Guan C, Zou T, Qiao Z, Duan S, Xie L, Yu Y, Zhang Y, Xu B, Ge J. Prognostic Implications of Prestent Pullback Pressure Gradient and Poststent Quantitative Flow Ratio in Patients Undergoing Percutaneous Coronary Intervention. J Am Heart Assoc 2022;11:e024903. [Crossref] [PubMed]
- Li C, Leng X, He J, Xia Y, Jiang W, Pan Y, Dong L, Sun Y, Hu X, Wang J, Xiang J, Jiang J. Diagnostic Performance of Angiography-Based Fractional Flow Reserve for Functional Evaluation of Coronary Artery Stenosis. Front Cardiovasc Med 2021;8:714077. [Crossref] [PubMed]
- Ueno K, Morita N, Kojima Y, Kondo H, Takahashi H, Minatoguchi S, Higuchi S, Ando Y, Esaki M. Efficacy of Low-Pressure Inflation of Oversized Drug-Coated Balloon for Coronary Artery Disease. J Interv Cardiol 2020;2020:6615988. [Crossref] [PubMed]
- van Zandvoort LJC, Masdjedi K, Witberg K, Ligthart J, Tovar Forero MN, Diletti R, Lemmert ME, Wilschut J, de Jaegere PPT, Boersma E, Zijlstra F, Van Mieghem NM, Daemen J. Explanation of Postprocedural Fractional Flow Reserve Below 0.85. Circ Cardiovasc Interv 2019;12:e007030. [Crossref] [PubMed]
- Baranauskas A, Peace A, Kibarskis A, Shannon J, Abraitis V, Bajoras V, Bilkis V, Aidietis A, Laucevicius A, Davidavicius G. FFR result post PCI is suboptimal in long diffuse coronary artery disease. EuroIntervention 2016;12:1473-80. [Crossref] [PubMed]
- Wang LL, Xu JP, He Y, Wang H, Zhao GZ, Wu K, He YM. Coronary Artery Tree Description and Lesion Evaluation (CatLet) score for functional evaluation of coronary stenosis: a comparison study with pressure wire fractional flow reserve. Quant Imaging Med Surg 2024;14:2857-69. [Crossref] [PubMed]
- Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med 2017;376:1813-23. [Crossref] [PubMed]
- Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med 2017;376:1824-34. [Crossref] [PubMed]
- Tonino PA, Johnson NP. Why Is Fractional Flow Reserve After Percutaneous Coronary Intervention Not Always 1.0? JACC Cardiovasc Interv 2016;9:1032-5. [Crossref] [PubMed]
- Zhou Z, Zhu B, Fan F, Yang F, Fang S, Wang Z, Qiu L, Gong Y, Huo Y. Prognostic Value of Coronary Angiography-Derived Fractional Flow Reserve Immediately After Stenting. Front Cardiovasc Med 2022;9:834553. [Crossref] [PubMed]
- Biscaglia S, Tebaldi M, Brugaletta S, Cerrato E, Erriquez A, Passarini G, Ielasi A, Spitaleri G, Di Girolamo D, Mezzapelle G, Geraci S, Manfrini M, Pavasini R, Barbato E, Campo G. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc Interv 2019;12:2079-88. [Crossref] [PubMed]
- Lee JM, Choi G, Hwang D, Park J, Kim HJ, Doh JH, Nam CW, Na SH, Shin ES, Taylor CA, Koo BK. Impact of Longitudinal Lesion Geometry on Location of Plaque Rupture and Clinical Presentations. JACC Cardiovasc Imaging 2017;10:677-88. [Crossref] [PubMed]


