
A novel nomogram model for diagnosing isolated atrial functional mitral regurgitation in patients with significant functional mitral regurgitation
Introduction
Mitral regurgitation (MR) is the predominant form of valvular heart disease worldwide, affecting over 2% of the global population, with its prevalence escalating as age advances (1-3). It is characterized by retrograde blood flow from the left ventricle into the atrium during systole, and leads to ventricular volume overload and heart failure. Functional MR is an important subtype of MR with a structurally normal mitral valve, and is associated with excess morbidity and mortality (3). Traditionally, the etiology of functional MR was thought to be regional and/or global left ventricular (LV) dysfunction (4). However, recent studies have shown that many patients with MR do not exhibit LV dysfunction; rather, they often present with an extremely enlarged left atrium (5). This type of MR is referred to as atrial functional mitral regurgitation (AFMR), and a higher prevalence has been observed throughout the world. Additionally, 27% of individuals with clinically significant (moderate or severe) MR have AFMR (6), and the 3-year mortality rate of these individuals has been reported to be as high as 41% (7).
The treatment strategies for isolated AFMR are quite different from those for traditional ventricular functional mitral regurgitation (VFMR). The etiology of AFMR is primarily linked to atrial fibrillation (AF) and heart failure with preserved ejection fraction (HFpEF) (8), which contribute to chronic left atrial (LA) dilation, dysfunction, and inadequate coaptation of the mitral leaflets. Due to its distinct pathology, the management of AFMR primarily involves rhythm control and mitral valvuloplasty rather than heart failure-related medical therapy (5). Therefore, the early identification of AFMR is extremely important.
Many similarities exist between these two types of functional MR; for example, VFMR patients may also experience AF and diastolic heart failure, while those with severe AFMR may experience significant heart failure, which complicates the diagnostic process (9). Further, some patients may exhibit features of both VFMR and AFMR, necessitating simultaneous treatment. Recently, several cases of typical echocardiographic features associated with AFMR have been reported (10,11). However, these features are difficult to quantify and are applied widely in clinical settings. Even in the latest guidelines and expert consensuses on valvular heart disease, the diagnostic criteria for AFMR remain ambiguous and lack a detailed and practical definition (1,2). Therefore, it is imperative to establish a new and simple approach that integrates clinical characteristics and echocardiographic parameters to accurately diagnose isolated AFMR.
A nomogram, based on multiple variables, is a common and reliable tool used to diagnose and estimate the prognosis of various diseases. Nomograms generate a numerical probability of a clinical event with rapid computation through friendly digital interfaces, and aid in clinical medical personalized decision making (12). Therefore, we evaluated the risk factors associated with isolated AFMR in a cohort of patients with significant functional MR and developed a predictive nomogram model to quantify the probability of AFMR to help cardiologists accurately diagnose AFMR and determine appropriate management strategies. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2913/rc).
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University (No. SYSKY-2022-299-03), and the requirement of individual consent for this retrospective analysis was waived.
Patients
This study conducted a retrospective analysis of the data of MR patients hospitalized at the Sun Yat-sen Memorial Hospital of Sun Yat-sen University from January 2020 to May 2023. The medical histories and clinical characteristics of the patients were extracted directly from the hospital’s electronic records.
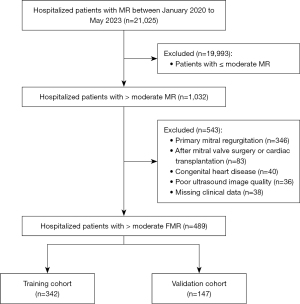
Patients were included in the study if they met the following inclusion criteria: (I) were aged ≥18 years; (II) had undergone transthoracic echocardiography showing moderate-to-severe or severe MR during hospitalization; and (III) had structurally normal mitral leaflets as confirmed by echocardiography. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had organic mitral valve disease, such as mitral prolapse, chordae tendineae rupture, rheumatic mitral disease, or infective endocarditis; (II) had previously undergone mitral valve surgery, or transcatheter/percutaneous mitral valve intervention; (III) had previously undergone cardiac transplantation; (IV) had congenital heart disease; (V) had echocardiography images of insufficient quality for analysis; and/or (VI) had incomplete clinical data. As Figure 1 shows, ultimately, 489 patients with significant functional MR were selected from a total of 21,025 MR patients. The patients were then randomly assigned to the training and validation cohorts at a ratio of 7:3.
Clinical and echocardiographic data
The clinical background information of the patients, including gender, age, relevant comorbidities, daily medications, and laboratory indexes, was obtained from the electronic records of the hospital. The history of AF was assessed by manual review of all resting electrocardiograms, 24-hour Holter monitoring, or charts before the index examination. Coronary artery disease, including myocardial angina, myocardial infarction (MI), and ischemic heart disease, was assessed by coronary arteriography, coronary computed tomography angiography, or previous diagnoses via chart review.
Echocardiographic examinations were conducted as part of routine clinical practice using a variety of commercially available machines at our institution. Measurements were guided by clinical recommendations for the assessment of cardiac cavities and regurgitation (13). The left atrial diameter (LAd) at end-systole and left ventricular diastolic diameter (LVDd) were assessed in the parasternal long-axis view. The left ventricular ejection fraction (LVEF) was obtained using the modified Simpson’s method. Additionally, peak tricuspid regurgitation velocity (TRv), which represents pulmonary pressure, was also recorded. The left ventricular (LV)/LA diastolic diameter ratio was calculated. According to the guidelines, MR was graded as mild (grade 1), moderate (grade 2), moderate-to-severe (grade 3), or severe (grade 4) based on combined qualitative, semiquantitative, and quantitative evaluations (14).
Two cardiologists with specialized expertise in echocardiography reviewed the clinical and echocardiographic data, and independently determined the types of MR. Functional MR was defined as MR without mitral valve impairment. All the functional MR patients were classified as isolated AFMR or non-isolated AFMR patients. The following criteria were used to diagnose isolated AFMR: (I) structurally normal mitral leaflets; (II) LA enlargement (a LA volume index >34 mL/m2, or a LAd >40 mm in men, or a LAd >38 mm in women) and mitral annular dilation (a mitral annular systolic anteroposterior diameter ≥35 mm); (III) a left ventricle cavity size within normal limits (a LVDd <55 mm) or exhibiting only mild dilation in patients with extremely severe chronic MR; (IV) a LVEF >45% and no regional ventricular myocardial wall motion abnormalities; (V) a loss of mitral leaflet concavity toward the left ventricle in the systole (Figure 2) (5,15,16). In cases of diagnostic inconsistency, the cardiologists consulted and discussed the issue first, and if a consensus could not be reached, the patient was subsequently excluded from the study.

This study adhered to a pre-defined protocol to ensure the rigor and reproducibility of the research. The research protocol and details of the availability of the study are available from the corresponding author on reasonable request. This study was not registered.
Statistical analysis
All the statistical analyses were performed using R4.0.2 statistical software. In this study, peak TRv, right ventricular (RV) transverse diameter, NT-proBNP, and serum creatinine had missing data rates of 8.8%, 3.3%, 3.9%, and 0.2%, respectively. The random forest method was employed for multiple imputation. The random forest method is capable of effectively handling non-linear relationships and complex data structures, ensuring the accuracy of the imputed values. The original data can be found in Tables S1-S3. The measurement data were tested for normality, and those that met the normal distribution are expressed as the mean ± standard deviation, and comparisons between the two groups were made using the independent samples t-test; those that did not meet the normal distribution are expressed as the median (25th–75th), and comparisons between the two groups were made using the Mann-Whitney U test. The count data are expressed as the case (%), and comparisons between groups were made using the chi-square test or Fisher’s exact test. Univariate and multivariate logistic regression analyses using the forward likelihood ratio method were performed to identify the independent predictors of isolated AFMR. A nomogram model for AFMR was developed based on factors from the multivariable logistic regression. The calibration curve was obtained using the bootstrap self-sampling method (of 1,000 bootstrap re-samples) to assess the predictive stability of this model and prevent over-fitting. A clinical decision curve analysis was conducted to evaluate the clinical usefulness of the established prediction model. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
In total, 489 patients (66.91±13.98 years old, 36.4% female) were included in this study. The demographic and clinical characteristics of the patients are shown in Table 1. In this cohort, 113 patients were diagnosed with isolated AFMR (the AFMR group), while 376 were diagnosed with non-isolated AFMR, including VFMR or other mixed types of functional MR (the non-AFMR group). The patients were significantly older (70.81±14.24 vs. 65.74±13.70 years, P<0.05), and the proportion of females was higher (56.6% vs. 30.3%, P<0.05) in the AFMR group than the non-AFMR group. There were more patients with AF (n=92, 81.4%) but fewer with MI (n=2, 1.8%) in the AFMR group than the non-AFMR group (all P<0.05). The NT-proBNP and serum creatinine levels were lower in the AFMR group than the non-AFMR group. In terms of the cardiac cavity dimensions, the AFMR patients had a larger LAd [47.0 (44.0–52.0) vs. 45.0 (42.0–49.0) mm, P<0.05] and a smaller LVDd [50.0 (47.0–54.0) vs. 63.0 (58.0–68.5) mm, P<0.05] than the non-AFMR patients. The AFMR patients had a better LVEF than the non-AFMR patients [62.0% (58.0–67.0%) vs. 36.0% (29.0–45.5%), P<0.05].
Table 1
| Variables | Isolated AFMR group (n=113) | Non-isolated AFMR group (n=376) | P value |
|---|---|---|---|
| Age (years) | 70.81±14.24 | 65.74±13.70 | 0.001 |
| Female | 64 (56.6) | 114 (30.3) | <0.001 |
| LAd (mm) | 47.0 (44.0–52.0) | 45.0 (42.0–49.0) | <0.001 |
| LVDd (mm) | 50.0 (47.0–54.0) | 63.0 (58.0–68.5) | <0.001 |
| LV/LA diastolic diameter ratio | 1.05±0.13 | 1.38±0.21 | <0.001 |
| LVEF (%) | 62.0 (58.0–67.0) | 36.0 (29.0–45.5) | <0.001 |
| Peak TRv (cm/s) | 289.86±39.36 | 295.32±51.15 | 0.296 |
| RA transverse diameter (mm) | 46.0 (43.0–51.0) | 43.0 (38.0–47.0) | <0.001 |
| RV transverse diameter (mm) | 41.0 (36.0–44.5) | 40.0 (36.0–44.0) | 0.086 |
| Comorbidity | |||
| AF | 92 (81.4) | 97 (25.8) | <0.001 |
| CHD | 18 (15.9) | 177 (47.1) | <0.001 |
| MI | 2 (1.8) | 84 (22.3) | <0.001 |
| Hypertension | 56 (49.6) | 182 (48.4) | 0.914 |
| DM | 18 (15.9) | 107 (28.5) | 0.011 |
| Cerebral stroke | 19 (16.8) | 51 (13.6) | 0.477 |
| NT-proBNP (pg/mL) | 1,653.0 (766.1–4,012.0) | 4,590.0 (1,835.8–11,004.5) | <0.001 |
| Serum creatinine (μmol/L) | 91.0 (72.5–118.5) | 113.0 (86.3–159.5) | <0.001 |
| Medication | |||
| Beta-blocker | 58 (51.3) | 214 (56.9) | 0.347 |
| ACEI/ARB/ARNI | 30 (26.5) | 224 (59.6) | <0.001 |
| CCB | 32 (28.3) | 81 (21.5) | 0.170 |
| MRA | 45 (39.8) | 260 (69.1) | <0.001 |
| Diuretics | 45 (39.8) | 200 (53.2) | 0.017 |
| Cordarone | 13 (11.5) | 52 (13.8) | 0.631 |
Continuous variables are presented as mean ± standard deviation or median (25th–75th percentile). Categorical variables are presented as n (%). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AFMR, atrial functional mitral regurgitation; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CCB, calcium channel blocker; CHD, coronary heart disease; DM, diabetes mellitus; LA, left atrial; LAd, left atrial diameter; LV, left ventricular; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; RA, right atrial; RV, right ventricular; TRv, tricuspid regurgitation velocity.
At a ratio of 7:3, 342 patients were allocated to the training cohort and 147 patients were allocated to the validation cohort. There were no significant differences in the clinical, echocardiographic, and laboratory data between the two cohorts. Detailed data are provided in Table 2.
Table 2
| Variables | Training group (n=342) | Validation group (n=147) | P value |
|---|---|---|---|
| Age (years) | 66.67±14.31 | 67.48±13.21 | 0.099 |
| Female | 126 (36.8) | 52 (35.4) | 0.757 |
| Types of mitral regurgitation | 0.212 | ||
| Isolated AFMR | 81 (23.7) | 32 (21.8) | |
| Non-isolated AFMR | 261 (76.3) | 115 (78.2) | |
| LAd (mm) | 46.0 (42.0–50.0) | 46.0 (43.0–49.0) | 0.887 |
| LVDd (mm) | 60.0 (53.0–66.0) | 60.0 (54.0–69.0) | 0.393 |
| LV/LA diastolic diameter ratio | 1.30±0.23 | 1.33±0.25 | 0.219 |
| LVEF (%) | 41.0 (30.8–57.0) | 40.0 (30.0–55.0) | 0.342 |
| Peak TRv (cm/s) | 295.41±48.68 | 290.89±48.77 | 0.843 |
| RA transverse diameter (mm) | 43.0 (39.0–47.0) | 44.0 (39.0–48.0) | 0.521 |
| RV transverse diameter (mm) | 40.0 (36.0–45.0) | 39.0 (36.0–43.0) | 0.150 |
| Comorbidity | |||
| AF | 127 (37.1) | 62 (42.2) | 0.294 |
| CHD | 138 (40.4) | 57 (38.8) | 0.106 |
| MI | 65 (19.0) | 21 (14.3) | 0.209 |
| Hypertension | 175 (51.2) | 63 (42.9) | 0.092 |
| DM | 90 (26.3) | 35 (23.8) | 0.560 |
| Cerebral stroke | 49 (14.3) | 21 (14.3) | 0.990 |
| NT-proBNP (pg/mL) | 3,659.0 (1,449.3–8,298.5) | 3,983.0 (1,310.0–9,729.2) | 0.918 |
| Serum creatinine (μmol/L) | 110.9 (82.0–154.0) | 100.0 (83.0–142.0) | 0.304 |
| Medication | |||
| Beta-blocker | 196 (57.3) | 76 (51.7) | 0.252 |
| ACEI/ARB/ARNI | 173 (50.6) | 81 (55.1) | 0.359 |
| CCB | 84 (24.6) | 29 (19.7) | 0.245 |
| MRA | 211 (61.7) | 94 (63.9) | 0.638 |
| Diuretics | 173 (50.6) | 72 (49.0) | 0.745 |
| Cordarone | 47 (13.7) | 18 (12.2) | 0.655 |
Continuous variables are presented as mean ± standard deviation or median (25th–75th percentile). Categorical variables are presented as n (%). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AFMR, atrial functional mitral regurgitation; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CCB, calcium channel blocker; CHD, coronary heart disease; DM, diabetes mellitus; LA, left atrial; LAd, left atrial diameter; LV, left ventricular; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; RA, right atrial; RV, right ventricular; TRv, tricuspid regurgitation velocity.
Independent risk factors for AFMR
The results of the univariate and multivariate logistic regression analyses are presented in Table 3. The univariate logistic regression analysis showed that age, female sex, previous AF, MI, diabetes mellitus, NT-proBNP, and serum creatinine were independent risk factors for AFMR. In relation to the echocardiographic data, the LAd, LVDd, LV/LA diastolic diameter ratio, LVEF, peak TRv, right atrial (RA) transverse diameter, and RV transverse diameter were also independent risk factors for AFMR. In the multivariate analysis, previous AF [odds ratio (OR): 9.34, 95% confidence interval (CI): 2.89–30.45, P<0.001], MI (OR: 0.04, 95% CI: 0.00–0.40, P=0.007), the LAd (OR: 1.14, 95% CI: 1.04–1.24, P=0.004), the LVDd (OR: 0.73, 95% CI: 0.65–0.82, P<0.001), and LVEF (OR: 1.21, 95% CI: 1.13–1.29, P<0.001) remained independently associated with AFMR.
Table 3
| Variables | Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|---|
| OR value | 95% CI | P value | OR value | 95% CI | P value | ||
| Age (years) | 1.03 | 1.01–1.05 | 0.004 | ||||
| Female | 0.36 | 0.22–0.60 | <0.001 | ||||
| LAd (mm) | 1.05 | 1.02–1.09 | 0.004 | 1.14 | 1.04–1.24 | 0.004 | |
| LVDd (mm) | 0.74 | 0.69–0.79 | <0.001 | 0.73 | 0.65–0.82 | <0.001 | |
| LV/LA diastolic diameter ratio | 0.00 | 0.00–0.00 | <0.001 | ||||
| LVEF (%) | 1.25 | 1.19–1.33 | <0.001 | 1.21 | 1.13–1.29 | <0.001 | |
| Peak TRv (cm/s) | 1.00 | 0.99–1.00 | 0.296 | ||||
| RA transverse diameter (mm) | 1.09 | 1.05–1.12 | <0.001 | ||||
| RV transverse diameter (mm) | 1.03 | 1.00–1.07 | 0.066 | ||||
| AF | 13.04 | 7.04–24.16 | <0.001 | 9.34 | 2.89–30.45 | <0.001 | |
| CHD | 0.28 | 0.15–0.51 | <0.001 | ||||
| MI | 0.08 | 0.01–0.26 | <0.001 | 0.04 | 0.00–0.40 | 0.007 | |
| Hypertension | 1.18 | 0.72–1.95 | 0.516 | ||||
| DM | 0.46 | 0.24–0.88 | 0.018 | ||||
| Cerebral stroke | 0.80 | 0.38–1.69 | 0.561 | ||||
| NT-proBNP (pg/mL) | 0.32 | 0.22–0.48 | <0.001 | ||||
| Serum creatinine (μmol/L) | 1.00 | 1.00–1.00 | 0.183 | ||||
AF, atrial fibrillation; AFMR, atrial functional mitral regurgitation; CHD, coronary heart disease; CI, confidence interval; DM, diabetes mellitus; LA, left atrial; LAd, left atrial diameter; LV, left ventricular; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio; RA, right atrial; RV, right ventricular; TRv, tricuspid regurgitation velocity.
Nomogram model of AFMR
Based on the results of the multivariate logistic regression analysis, AF, MI, the LAd, the LVDd, and the LVEF were used to construct a nomogram model (Figure 3A). The scores for each independent risk factor were summed to calculate a total score. The results showed that the score derived from the LVDd and LVEF contributed the most to the total score, and the score was higher when the LVDd was smaller and the LVEF was better. Although the patients with AF exhibited a greater likelihood of AFMR, the presence of AF only had a relatively minor effect on the total score. Eventually, this total score was used to generate an individual numerical probability of AFMR. For example, a 78-year-old male patient, who had a history of AF but no history of MI, and a LAd of 50 mm, a LVDd of 55 mm, and a LVEF of 60% based on echocardiography, had a total score of 137 points, which corresponded to a 92% probability of isolated AFMR. This patient was comprehensively evaluated by two senior cardiologists based on imaging and clinical data, and AFMR was confirmed. The calculation formula was as follows: 12.5+75+28+8+13.5=137 points (Figure 3B).

Evaluation and validation of the nomogram
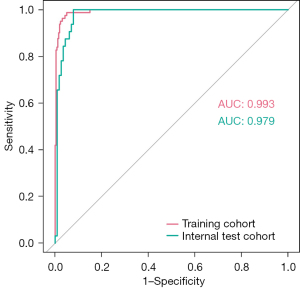
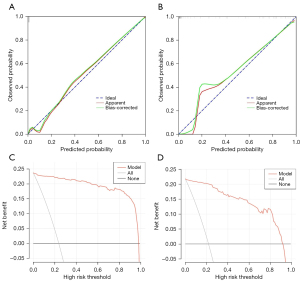
The area under the curve (AUC) values were 0.993 and 0.979 in the training and validation cohorts, respectively; thus, the nomogram showed excellent predictive ability (Figure 4). The bootstrap validation method was used to validate this model internally. The calibration curve showed good concordance between the predicted and observed values in the training and validation cohorts. The final decision curve analysis showed that for a threshold probability between 0% and 97%, the model had a positive net benefit (Figure 5).
Discussion
In the current study, we developed and validated a nomogram model to assess the individual probability of isolated AFMR using a cohort of 489 patients with functional MR. The main predictors incorporated into the nomogram included the LAd, LVDd, LVEF, AF, and MI, which were statistically significant in the multivariate logistic regression analysis. In terms of its discriminative performance, the model performed well in the training and test cohorts. To the best of our knowledge, no similar studies have been conducted previously.
AFMR is a distinct subtype of functional MR that has garnered increased attention in recent years due to its increasing prevalence and recognition of the condition. Its complex pathophysiology suggests that AFMR requires different management strategies than other functional MR, and patients might derive benefits from treatments targeting the underlying arrhythmia and valvular pathology. Research by Hirji et al. revealed significant differences in baseline cardiac morphology and function between AFMR and VFMR patients. These differences clearly affect in-hospital and mid-term outcomes (16). Therefore, the timely and accurate identification of AFMR is extremely important.
The diagnostic criteria of current clinical trials vary widely. First, several studies have used the mere presence of AF to define AFMR cohorts, while excluding patients with HFpEF (17-19). However, patients with functional MR induced by ventricular impairment may also have long-standing AF that could be misdiagnosed as AFMR. Second, AFMR is typically observed in patients with normal LV function, with LV dysfunction serving as an exclusion criterion. However, in the valvular heart disease guidelines of Europe and the United States, the LVEF cutoff value for distinguishing AFMR from VFMR remains unclear, while LVEF cutoff values in various studies range from 45% to 55%. Third, some researchers have suggested that AFMR should be diagnosed based on certain echocardiographic features, such as a central MR jet, seagull sign, mitral annular dilation, reduced mitral annular contractility, and abnormal annual-papillary balance (10,11). However, the widespread application of these features in clinical practice is fraught with difficulties, as these features are not AFMR-specific features and can also be present in non-AFMR patients. Additionally, they are highly operator-dependent and only used by specialized cardiologists with expertise in echocardiography. Most importantly, no clear cut-off values for these features have been reported (20). Thus, there is a pressing need for a more individual, specific, and reliable approach for identifying AFMR, for which the nomogram model has emerged as one of the most promising methods.
In the present study, we analyzed the characteristics of AFMR and observed that the affected individuals were predominantly elderly females who had a history of AF but no prior MI, and lower NT-proBNP and serum creatinine levels. When we included all the clinical, laboratory, and echocardiogram data into the univariate and multivariable analyses, only previous AF, MI, the LAd, the LVDd, and the LVEF were predictors of isolated AFMR, and were thus included in the nomogram model.
Given that LA-related myopathy is the most important etiological factor for AFMR, it is not surprising that AF and the LAd are the two principal predictors of this condition. Abe et al. found that as the duration of AF increased, there was a significant increase in the incidence of severe MR (from 8.1% in 1–10 years to 28% in more than 10 years) (21). Gertz et al. reported that the enlargement of the left atrium and mitral annulus, which is caused by AF, was the primary pathophysiological mechanism underlying AFMR (22). However, in our study, we found that the weight score of AF was not prominent in the diagnostic model, while the LAd exerted a more robust influence. In AFMR, LA dilation has emerged as a paramount upstream pathogenic factor for MR. This dilation triggers atriogenic leaflet tethering, anomalies in mitral annular remodeling (23), and maladaptive leaflet growth, culminating in insufficient coaptation of the mitral leaflets and exacerbating the severity of MR (5). Nevertheless, diseases other than AF (e.g., HFpEF) have the potential to precipitate LA dilation and fibrosis (24-26). Consequently, an increased LAd, as opposed to the mere presence of AF, indicates a more heightened risk for AFMR.
In addition to the parameters mentioned above, it was observed that LV-related parameters, specifically the LVDd and LVEF, were the most influential risk factors for AFMR. In contrast to VFMR, which is instigated by LV remodeling and systolic dysfunction, the left ventricles in AFMR are nearly normal. Therefore, patients with significant MR but normal LV function, particularly those with a relatively small LVDd, are more likely to have isolated AFMR. Similarly, the 2022 Journal of the American College of Cardiology (JACC) Expert Consensus on AFMR Imaging described the features of AFMR as follows: “The LVEF is preserved, and the LV size remains within the normal range in the early stage, but may increase in the late stage” (15). However, establishing definitive LVEF and LVDd cut-off values for AFMR is challenging, and these values vary across different guidelines and clinical trials (5,26). Instead of relying on specific numerical cut-off values, we converted the measured values of LVEF and LVDd into scores and provided individualized probabilities for AFMR. Patients with a smaller LVDd and a higher LVEF had a much higher probability of AFMR.
In the present study, the absence of a history of MI was identified as an independent risk factor for AFMR. MI instigates local myocardial fibrosis and dysfunction, and intricate LV geometric modifications, and triggers pathological alterations in the valve and sub-valvular apparatus, particularly in cases of posterior MI, ultimately culminating in significant VFMR (27). Thus, patients without a history of MI are more likely to have AFMR.
Several variables (i.e., age, female gender, RA transverse diameter, and NT-proBNP) were excluded from the final model. However, the disparities between the groups retained substantial clinical significance. The study delineated certain clinical features of patients with AFMR. Specifically, in comparison to the patients with non-isolated AFMR, the isolated-AFMR patients were more frequently elderly females, displayed relatively lower NT-proBNP levels, and exhibited relatively larger right atria. In relation to the factor of age, a direct correlation was found between advancing age and an increased incidence of AF (28). Given that AF is one of the primary etiologies of AFMR, age may be an indirect contributing factor to the development of AFMR. In terms of gender, Stoicescu et al. reported a pronounced gender disparity among patients with HFpEF, such that the female patients significantly outnumbered the male patients at a ratio of approximately 2:1 (29). As HFpEF patients are also a major cohort susceptible to AFMR, gender may play a role in the pathogenesis of AFMR. NT-proBNP is a well-established biomarker indicative of cardiac function, which is much higher in VFMR patients. However, the LVDd and LVEF are two parameters that can assess LV structure and contractility more directly, and thus effectively supplanted NT-proBNP in representing cardiac function in the nomogram model.
Among the isolated AFMR cases, 18.6% of patients had no history of AF. These results are highly consistent with those of Kagiyama et al., who reported that the proportion was 19.9% (30). The causes of LA enlargement in these patients include HFpEF, atrial cardiomyopathy, hypertension, and other factors, with HFpEF being the main cause. In HFpEF patients, the diastolic function of the left ventricle is reduced, leading to increased LV filling pressure, which in turn causes a persistent rise in LA pressure. To overcome the resistance during blood ejection, the left atrium gradually undergoes compensatory dilation. Concurrently, the mechanical function of the left atrium becomes impaired, leading to LA fibrosis and electrical remodeling. The dilation of the left atrium further contributes to the expansion of the mitral annulus, ultimately resulting in the development of AFMR (15).
The AUC value of the diagnostic nomogram was remarkably large, indicating its exceptional predictive capability. The decision curve analysis further showed that this nomogram model yielded greater net benefits in the clinical setting. This study was the first to establish a nomogram prediction model for AFMR in a population of patients with functional MR. Based on the results of the multivariate logistic regression analysis, this study combined five indicators (i.e., the LAd, LVDd, LVEF, AF, and MI). By using a scaled line segment, a total score was calculated according to the patients’ clinical and echocardiographic characteristics. A higher score corresponds to a greater probability, indicating a higher likelihood of being diagnosed with AFMR. This nomogram is easy to use and intuitive, and thus could help clinicians to conduct individualized assessments of the risk of AFMR in patients.
For AFMR patients, rhythm control, HFpEF-related therapy, and mitral valvuloplasty (either surgical or transcatheter repair) are of paramount importance (5,31,32). Conversely, for VFMR patients, heart failure guideline-directed medical therapy, including beta-blockers, renin-angiotensin system inhibition, sodium-glucose cotransporter 2 inhibitors, and mineralocorticoid receptor antagonists, and implantable cardioverter-defibrillator/cardiac resynchronisation therapy should be prioritized initially to reverse LV remodeling and increase systolic function (33-35). In cases of persistent severe MR, mitral valvuloplasty may be necessary (36-38). Thus, our nomogram model can be used to assess the probability of AFMR and is a valuable tool that cardiologists can use to evaluate the success rates of different therapies for functional MR.
This study had several limitations. First, it was a retrospective study, which potentially introduced selection bias. Second, our study exclusively incorporated two-dimensional parameters, such as the LAd and LVDd, and did not use volumetric parameters, particularly those derived from three-dimensional echocardiography. However, it should be noted that the LAd and LVDd are easily obtainable and have minimal operator dependence, and are also recommended by established guidelines for cardiac chamber quantification (13). Third, this was a single-center study; thus, future research efforts ought to involve multicenter clinical external validation data to enhance the generalizability and robustness of the findings.
Conclusions
For patients with functional MR, the timely and precise identification of AFMR is crucial in the clinic. This study devised a straightforward approach for diagnosing AFMR. Specifically, it established a nomogram model that integrates the LAd, LVDd, LVEF, AF, and MI. This nomogram could help cardiologists in treatment determination and prognosis evaluation.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2913/rc
Funding: This research was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2913/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University (No. SYSKY-2022-299-03), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed) 2022;75:524. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, Zühlke L, Prendergast BD. Global epidemiology of valvular heart disease. Nat Rev Cardiol 2021;18:853-64. [Crossref] [PubMed]
- Bartko PE, Heitzinger G, Spinka G, Pavo N, Prausmüller S, Kastl S, Winter MP, Arfsten H, Tan TC, Gebhard C, Mascherbauer J, Hengstenberg C, Strunk G, Hülsmann M, Goliasch G. Principal Morphomic and Functional Components of Secondary Mitral Regurgitation. JACC Cardiovasc Imaging 2021;14:2288-300. [Crossref] [PubMed]
- Farhan S, Silbiger JJ, Halperin JL, Zhang L, Dukkipati SR, Vogel B, Kini A, Sharma S, Lerakis S. Pathophysiology, Echocardiographic Diagnosis, and Treatment of Atrial Functional Mitral Regurgitation: JACC State-of-the-Art Review. J Am Coll Cardiol 2022;80:2314-30. [Crossref] [PubMed]
- Dziadzko V, Dziadzko M, Medina-Inojosa JR, Benfari G, Michelena HI, Crestanello JA, Maalouf J, Thapa P, Enriquez-Sarano M. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J 2019;40:2194-202. [Crossref] [PubMed]
- Mesi O, Gad MM, Crane AD, Ramchand J, Puri R, Layoun H, Miyasaka R, Gillinov MA, Wierup P, Griffin BP, Kapadia SR, Harb SC. Severe Atrial Functional Mitral Regurgitation: Clinical and Echocardiographic Characteristics, Management and Outcomes. JACC Cardiovasc Imaging 2021;14:797-808. [Crossref] [PubMed]
- Moonen A, Ng MKC, Playford D, Strange G, Scalia GM, Celermajer DS. Atrial functional mitral regurgitation: prevalence, characteristics and outcomes from the National Echo Database of Australia. Open Heart 2023;10:e002180. [Crossref] [PubMed]
- Reid A, Ben Zekry S, Naoum C, Takagi H, Thompson C, Godoy M, Anastasius M, Tarazi S, Turaga M, Boone R, Webb J, Leipsic J, Blanke P. Geometric differences of the mitral valve apparatus in atrial and ventricular functional mitral regurgitation. J Cardiovasc Comput Tomogr 2022;16:431-41. [Crossref] [PubMed]
- Kim DH, Heo R, Handschumacher MD, Lee S, Choi YS, Kim KR, Shin Y, Park HK, Bischoff J, Aikawa E, Song JM, Kang DH, Levine RA, Song JK. Mitral Valve Adaptation to Isolated Annular Dilation: Insights Into the Mechanism of Atrial Functional Mitral Regurgitation. JACC Cardiovasc Imaging 2019;12:665-77. [Crossref] [PubMed]
- Okamoto H, Miyake M, Hayashi A, Matsutani H, Tamura T, Nakagawa Y. Differences in clinical and echocardiographic features and outcomes between atrial functional mitral regurgitation patients with and without posterior mitral leaflet bending. J Cardiol 2023;82:22-8. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Zoghbi WA, Levine RA, Flachskampf F, Grayburn P, Gillam L, Leipsic J, Thomas JD, Kwong RY, Vandervoort P, Chandrashekhar Y. Atrial Functional Mitral Regurgitation: A JACC: Cardiovascular Imaging Expert Panel Viewpoint. JACC Cardiovasc Imaging 2022;15:1870-82. [Crossref] [PubMed]
- Hirji SA, Cote CL, Javadikasgari H, Malarczyk A, McGurk S, Kaneko T. Atrial functional versus ventricular functional mitral regurgitation: Prognostic implications. J Thorac Cardiovasc Surg 2022;164:1808-1815.e4. [Crossref] [PubMed]
- Matsumori M, Kawashima M, Aihara T, Fujisue J, Fujimoto M, Fukase K, Nomura Y, Tanaka H, Murakami H, Mukohara N. Efficacy of left atrial plication for atrial functional mitral regurgitation. Gen Thorac Cardiovasc Surg 2021;69:458-65. [Crossref] [PubMed]
- Kaneyuki D, Nakajima H, Asakura T, Yoshitake A, Tokunaga C, Tochii M, Hayashi J, Takazawa AT, Izumida H, Iguchi A. The change in the mitral-septal angle after surgery for atrial functional mitral regurgitation. Gen Thorac Cardiovasc Surg 2021;69:1-7. [Crossref] [PubMed]
- Carino D, Lapenna E, Ascione G, Ruggeri S, Del Forno B, Castiglioni A, Alfieri O, De Bonis M. Is mitral annuloplasty an effective treatment for severe atrial functional mitral regurgitation? J Card Surg 2021;36:596-602. [Crossref] [PubMed]
- Doldi P, Stolz L, Orban M, Karam N, Praz F, Kalbacher D, et al. Transcatheter Mitral Valve Repair in Patients With Atrial Functional Mitral Regurgitation. JACC Cardiovasc Imaging 2022;15:1843-51. [Crossref] [PubMed]
- Abe Y, Akamatsu K, Ito K, Matsumura Y, Shimeno K, Naruko T, Takahashi Y, Shibata T, Yoshiyama M. Prevalence and Prognostic Significance of Functional Mitral and Tricuspid Regurgitation Despite Preserved Left Ventricular Ejection Fraction in Atrial Fibrillation Patients. Circ J 2018;82:1451-8. [Crossref] [PubMed]
- Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol 2011;58:1474-81. [Crossref] [PubMed]
- Akamatsu K, Abe Y, Matsumura Y, Shimeno K, Naruko T, Takahashi Y, Shibata T, Yoshiyama M. Etiology of Atrial Functional Mitral Regurgitation: Insights from Transthoracic Echocardiography in 159 Consecutive Patients with Atrial Fibrillation and Preserved Left Ventricular Ejection Fraction. Cardiology 2020;145:511-21. [Crossref] [PubMed]
- Citerni C, Kirchhoff J, Olsen LH, Sattler SM, Gentilini F, Forni M, Zannoni A, Grunnet M, Edvardsson N, Bentzen BH, Diness JG. Characterization of Atrial and Ventricular Structural Remodeling in a Porcine Model of Atrial Fibrillation Induced by Atrial Tachypacing. Front Vet Sci 2020;7:179. [Crossref] [PubMed]
- Bonow RO, O'Gara PT, Adams DH, Badhwar V, Bavaria JE, Elmariah S, Hung JW, Lindenfeld J, Morris AA, Satpathy R, Whisenant B, Woo YJ. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;75:2236-70. [Crossref] [PubMed]
- Amabile A, Fereydooni S, Mori M, Hameed I, Jung J, Krane M, Geirsson A. Variable definitions and treatment approaches for atrial functional mitral regurgitation: A scoping review of the literature. J Card Surg 2022;37:1182-91. [Crossref] [PubMed]
- Nogara A, Minacapelli A, Zambelli G. V LC, Fattouch K. Functional anatomy and echocardiographic assessment in secondary mitral regurgitation. J Card Surg 2022;37:4103-11. [Crossref] [PubMed]
- Wang L, Ze F, Li J, Mi L, Han B, Niu H, Zhao N. Trends of global burden of atrial fibrillation/flutter from Global Burden of Disease Study 2017. Heart 2021;107:881-7. [Crossref] [PubMed]
- Stoicescu L, Crişan D, Morgovan C, Avram L, Ghibu S. Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. Int J Mol Sci 2024;25:794. [Crossref] [PubMed]
- Kagiyama N, Kaneko T, Amano M, Sato Y, Ohno Y, Obokata M, et al. Clinical Outcomes of Mitral Valve Surgery in Atrial Functional Mitral Regurgitation in the REVEAL-AFMR Registry. JAMA Netw Open 2024;7:e2428032. [Crossref] [PubMed]
- Abe Y, Takahashi Y, Shibata T. A new disease entity: Atrial functional mitral regurgitation. J Cardiol 2021;77:565-9. [Crossref] [PubMed]
- Deferm S, Bertrand PB, Verhaert D, Verbrugge FH, Dauw J, Thoelen K, Giesen A, Bruckers L, Rega F, Thomas JD, Levine RA, Vandervoort PM. Mitral Annular Dynamics in AF Versus Sinus Rhythm: Novel Insights Into the Mechanism of AFMR. JACC Cardiovasc Imaging 2022;15:1-13. [Crossref] [PubMed]
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895-e1032. [Crossref] [PubMed]
- Coats AJS, Anker SD, Baumbach A, Alfieri O, von Bardeleben RS, Bauersachs J, et al. The management of secondary mitral regurgitation in patients with heart failure: a joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur Heart J 2021;42:1254-69. [Crossref] [PubMed]
- Milwidsky A, Mathai SV, Topilsky Y, Jorde UP. Medical Therapy for Functional Mitral Regurgitation. Circ Heart Fail 2022;15:e009689. [Crossref] [PubMed]
- Yoon SH, Makar M, Kar S, Chakravarty T, Oakley L, Sekhon N, Koseki K, Nakamura M, Hamilton M, Patel JK, Singh S, Skaf S, Siegel RJ, Bax JJ, Makkar RR. Outcomes After Transcatheter Edge-to-Edge Mitral Valve Repair According to Mitral Regurgitation Etiology and Cardiac Remodeling. JACC Cardiovasc Interv 2022;15:1711-22. [Crossref] [PubMed]
- Izumi C, Eishi K, Ashihara K, Arita T, Otsuji Y, Kunihara T, et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ J 2020;84:2037-119. [Crossref] [PubMed]
- Goel K, Lindenfeld J, Makkar R, Naik H, Atmakuri S, Mahoney P, Morse MA, Thourani VH, Yadav P, Batchelor W, Rogers J, Whisenant B, Rinaldi M, Hermiller J, Lindman BR, Barker CM. Transcatheter Edge-to-Edge Repair in 5,000 Patients With Secondary Mitral Regurgitation: COAPT Post-Approval Study. J Am Coll Cardiol 2023;82:1281-97. [Crossref] [PubMed]


